2025年9月22日,FDA 发布了 CohanceLimited Lifesciences的483缺陷报告,其中提及缺陷如下:
一批被决定销毁的不合格片剂,在仓库中部分失踪。公司QA总经理和运营高级总经理无法解释数量差异,且没有关于这些物料去向的处置和核对文件。从2023年8月被拒到2025年8月检查当日,近23个月未处理。
对一次OOS含量测定不合格的调查结论草率。被简单归咎于“操作员可能遗漏步骤”,但未评估和解决其背后“操作员假设主管不在场即可跳过步骤”这一严重的流程和操作漏洞,也未采取预防措施。
决定更换取样小瓶类型以消除称重误差,但未评估原小瓶材料是否对检测结果有影响,未进行假设检验。
对直接接触药品的包装容器未进行可提取物和浸出物研究。公司无法提供任何相关研究文件,无法保证包装材料不会影响药品的安全性和质量。
对关于片剂在瓶内碎裂、产生粉尘的投诉,调查结论为“不成立”,且未采取任何行动。未建立成品放行标准来界定片剂边缘碎裂、崩解等物理特性的可接受限度。检查中在留样样品上也观察到同样问题,但未进行深入评估,如索取原始对照样品比对。根据内部SOP,此类情况应上报FDA现场警报报告,但未执行且无任何解释。
针对产品中发现手套碎片的投诉,新制定的《手套处理SOP》未包含要求员工在发现手套破损时通知主管或记录事件的条款,无法防止未来类似事件的发生。
瓶内片剂规格混淆的投诉应记录到现场警报报告日志,但是未被记录。QA部门以“对程序不了解”为由,未遵守SOP QA084-02的要求。
仪器使用日志缺失或不完整,原因再次归咎于“操作员和QA对程序不了解”;关键质量相关仪器没有或未按要求填写使用日志。分析天平#PD/AWB-06没有使用日志;脆碎度测试仪PD/FBL-01的使用日志未按SOP要求填写。
生产设备访问权限控制不足,生产设备面板(HMI)共享高级别用户名和密码,且无风险评估。操作员被授予了本应仅限主管知晓的密码,该权限允许他们编辑循环时间、设定点等关键工艺参数。这违反了设备供应商手册的规定,且公司没有对此安排进行书面风险评估。
包装主管可以且确实删除了包装线计算机系统中的审计追踪和批次数据文件。该系统的登录账户也在操作员和主管间共享,IT管理员承认此为“疏忽”。
在设备PD ×× 01内部观察到可见污渍,擦拭取样检测出前序产品的残留;胶囊填充机PD/CFM-03上观察到可见的产品残留,但清洁记录显示已完成清洁,检查时的擦拭取样结果证实清洁无效。
压片机PD/TCM-03存在多项维护问题,设备上有污渍、凹痕、部件损坏/碎裂、金属颗粒,存在污染产品和影响片剂质量的风险。
关键设备出现裂纹,但未记录事件、未进行评估、也未进行维护。
引入多个新产品未纳入清洁验证评估;仅用一种片剂代表所有剂型进行清洁验证;日常清洁中使用的清洁剂未包含在最初的清洁验证中。
设备部件出现在不应出现的生产区域,且无记录和解释;关键部件在批间断开连接,重新连接使用前无功能验证程序。
样品制备程序和允许的样品质量差异范围未进行评估。历史上多次OOS结果被归因于“取样误差”,但根本性的方法问题未得到解决。
具体缺陷内容如下:
OBSERVATION 1
观察项1
There is a failure to thoroughly review any unexplained discrepancy and the failure of a batch or any of its components to meet any of its specifications whether or not the batch has been already distributed.未能对任何无法解释的差异以及某一批次或其任何组件不符合任何规格要求的情况进行彻底审查,无论该批次是否已分发。
A) There is no documentation on the disposition status and reconciliation of approximately ×× of rejected ×× tablets, ×× mg, Batch No. ×× that have gone missing after being rejected on August 23, 2023, as part of Deviation DEV/NCR/23/0033, Deviation Initiation Date: June 19, 2023. Specifically, ×× total containers, which consists of ×× of Batch No. ×× compressed tablets, had fallen on the floor from the ×× during transfer from the hold area to Bottle packing line ××. According to the final investigation report, the decision was made to destroy the entire batch due to the high number of rejects after tablet reinspection and probable impact on physical characteristics for all compressed tablets. According to the General Manager of Quality Assurance (QA), the rejected batch has not been disposed of as of the current inspection; this is approximately 23 months since the final investigation report was closed and approved by the QA and Production department in August 2023. However, only ×× out of the ×× total containers, which consists of ×× out of the ×× total rejected batch material, remained in the warehouse facility during my inspection walkthrough on August 08, 2025. The General Manager of QA and Senior General Manager of Operations stated that they were unable to explain the discrepancy and unable to account for the missing quantity of rejected compressed tablets for Batch No. ×× which was initially intended for distribution to the U.S market.
关于于 2023 年 8 月 23 日被拒收后失踪的、属于偏差 DEV/NCR/23/0033(偏差发起日期:2023 年 6 月 19 日)的一部分的、约 ×× 的 ×× 毫克、批号为 ×× 的拒收片剂的处置状态和核对,没有相关文件记录。具体来说,在从暂存区转移到瓶装包装线 ×× 的过程中,包含批号为 ×× 的 ×× 片压片的 ×× 个总容器从 ×× 掉到了地上。根据最终调查报告,由于片剂复检后拒收数量众多,且可能对所有压片的物理特性产生影响,决定销毁整批产品。根据质量保证(QA)总经理的说法,截至本次检查,被拒收的批次尚未被处理;自 2023 年 8 月 QA 和生产部门关闭并批准最终调查报告以来,已经过去了大约 23 个月。然而,在我 2025 年 8 月 8 日的检查巡视中,只有 ×× 个总容器(包含 ×× 的总拒收批次物料)仍在仓库设施中。QA 总经理和运营高级总经理表示,他们无法解释这一差异,也无法说明最初拟分销到市场的批号为 ×× 的拒收压片的缺失数量的去向。
B) OOS/IP/21/020 was initiated on March 30, 2021, due to ×× Tablets USP, Batch No. ×× Mfg. Date: 03/2021, Exp. Date: ×× HPLC assay test not meeting specification limits for the ×× stage. The batch was initially intended for the U.S market but ultimately rejected due to the following root cause: Operator potentially missing steps for ×× and subsequently leading to inadequate ××. According to the investigation report personnel interview section, it was understood that the ×× step was possibly missed by the Operator due to assumption that the Executive (BMR Reviewer) being absent for verification. The investigation further stated that since the executed procedure was performed during ×× the assumption/overlook tendency of the operator and unavailability of operator for missing the step is inferred to be inherent. However, there was no further assessment and preventive actions initiated to address the process and operational gaps that may continue to remain during critical manufacturing and packaging steps (especially during ××).
OOS/IP/21/020 于 2021 年 3 月 30 日发起,原因是 ×× USP片剂,批号 ××,生产日期:2021 年 3 月,有效期:××,高效液相色谱(HPLC)含量测定测试在 ×× 阶段不符合规格限值。该批次最初拟用于美国市场,但最终因以下根本原因被拒收:操作员可能遗漏了 ×× 的步骤,进而导致 ×× 不充分。根据调查报告的人员访谈部分,了解到操作员可能由于认为主管(BMR 审核员)不在场无法进行验证,而遗漏了 ×× 步骤。调查进一步指出,由于在 ×× 期间执行了该程序,操作员的假设 / 疏忽倾向以及操作员因遗漏步骤而无法到场的情况被推断为固有存在的。然而,没有进一步开展评估和启动预防措施,以解决在关键生产和包装步骤(尤其是在 ×× 期间)可能持续存在的工艺和操作差距。
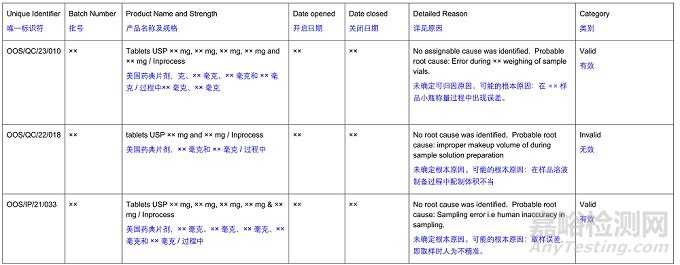
C) OOS/QC/23/010 was initiated on April 28, 2023, due to ×× Tablets USP ×× mg, ×× mg, ×× mg, ×× mg and ×× mg /In-process, Batch No. ×× having out-of-specification (OOS) results for the mean of individual assay: ×× % (Specification: ×× %) for Sample Set ×× Sample Set ×× had passing results and no assignable root cause was identified for the Sample Set ×× OOS results. As a preventive action, it was proposed to collect the ×× content assay samples at ×× stage in ×× vials (instead of ×× vials) to eliminate error during ×× weighing of sample vials. However, no further hypothesis testing, or detailed assessment was performed to evaluate the effects that ×× material may have on altering sample results before switching to the usage of ×× vials.
OOS/QC/23/010 于 2023 年 4 月 28 日发起,原因是 ×× USP片剂,×× 毫克、×× 毫克、×× 毫克、×× 毫克和 ×× 毫克 / 过程中,批号 ×× 的单个含量测定平均值出现超出规格(OOS)的结果:××%(规格:××%)。样本组 ××、样本组 ×× 有合格结果,且未确定样本组 ××OOS 结果的可指定根本原因。作为预防措施,提议在 ×× 阶段用 ×× 小瓶(而非 ×× 小瓶)收集 ×× 含量测定样本,以消除在 ×× 样本小瓶称重过程中的误差。然而,在改用 ×× 小瓶之前,没有进行进一步的假设检验或详细评估,以评估 ×× 材料可能对改变样本结果产生的影响。
Compressed tablets and finished product capsules are then packaged into ×× bottles that are distributed to the U.S market. ×× Tablets ×× mg, final batch No. ×× Mfg. Date: April 2023 was ultimately released to the U.S market based on passing ×× of dosage unit results and Sample Set ×× testing results in June 2023 of ×× Tablets within ×× Bottles).
压片和成品胶囊随后被包装到 ×× 瓶中,分销到美国市场。×× 毫克的 ×× 片剂,最终批号 ××,生产日期:2023 年 4 月,最终基于 2023 年 6 月 ×× 瓶内 ×× 片剂的剂量单位结果合格以及样本组 ×× 测试结果合格,被放行到美国市场。
According to the Assistant General Manager of Quality Assurance, there is no documentation or record onsite for extractable and leachable studies being performed for ×× bottle containers used for ×× packaging of all tablets and capsules distributed to the US and other markets. During the current inspection, your firm was unable to provide any documentation of extractable and leachable studies performed for the ×× containers ×× for finished product tablet compression or capsule filling operations.
根据质量保证总经理助理的说法,现场没有关于对用于向美国和其他市场分销的所有片剂和胶囊的 ×× 包装的 ×× 瓶容器进行可萃取和可浸出研究的文件或记录。在本次检查中,贵公司无法提供任何关于对用于成品片剂压片或胶囊填充操作的 ×× 容器 ×× 进行可萃取和可浸出研究的文件。
OBSERVATION 2
观察项2
Written records of investigation of a drug complaint do not include the follow - up.
药品投诉调查的书面记录不包含后续跟进内容。
A) Complaint #PC/NCR/FDF/23 - 020, was filed on October 5th, 2023 for ×× tablets USP ×× mg, due to the firm's customer receiving a complaint from the pharmacy that the tablets were disintegrating and found the tablets in the bottle to be crumbly, disintegrating, pitted, had dust in the in the bottles that came from the pill itself and did not have crisp markings on the tablet. The investigation report root cause analysis stated that: based on the investigation, it can be drawn that the tablets photographs of the control/reference sample resemble the tablet photographs of the complaint sample. The probability of generation of powder could be due to ×× nature of the tablets. In addition, the subjected complaint was considered as 'Not Substantiated' and no corrective and/or preventive actions was initiated. However, there is no finished drug product release and stability specification established to determine the acceptability limit of ×× tablets with chipped edges and disintegrating like physical characteristics. These physical characteristics was also observed during review of retain samples during the current inspection. According to the General Manager of Quality Assurance (QA), there was also no attempt made to request the original comparator sample for comparison purposes and further evaluation.
投诉 #PC/NCR/FDF/23 - 020 于 2023 年 10 月 5 日提出,涉及 ×× 毫克的 ×× USP片剂。原因是该公司的客户收到药房投诉,称片剂正在崩解,且发现瓶中的片剂易碎、崩解、有凹痕,瓶中有来自药片本身的粉尘,并且片剂上没有清晰的标识。调查报告的根本原因分析表明:根据调查,对照 / 参考样品的片剂照片与投诉样品的片剂照片相似。产生粉末的可能性可能是由于片剂的 ×× 特性。此外,该投诉被认定为 “不成立”,且未启动任何纠正和 / 或预防措施。然而,没有制定成品药品的放行和稳定性规格来确定具有边缘碎裂和类似崩解等物理特性的 ×× 片剂的可接受限度。在本次检查期间对留样进行审查时,也观察到了这些物理特性。据质量保证(QA)总经理称,也没有尝试要求提供原始对照样品以作比较和进一步评估。
Furthermore, SOP QA084 - 02, Field Alert Report, Effective Date: December 31, 2022, states that: the following are some examples which need to be reported as a Field Alert Report (FAR): Crumbled, broken, disintegrated tablets found inside bottles...However, there is no documented explanation as to why there was no communication with the customer in regards to potentially filing a U.S FDA field alert report for the complaint as described in the procedure.
此外,SOP QA084 - 02《现场警戒报告》,生效日期:2022 年 12 月 31 日,规定:以下是一些需要作为现场警戒报告(FAR)上报的例子:瓶内发现碎裂、破损、崩解的片剂…… 然而,对于为何没有按照程序所述,就该投诉与客户沟通是否可能提交美国食品药品监督管理局(FDA)现场警戒报告,没有书面说明。
B) Complaint # PC/NCR/FDF/24 - 024 was initiated on May 10, 2024, for ×× Tablets, ×× mg, Batch No. ×× due to customer finding medical grade piece of glove, which is/could be a finger part of the glove, upon opening the sealed bottle on April 23, 2024. It was determined that the glove might have torn during ×× activity prior to tablet inspection and then ×× the tablets and filled in the CAPA/030/24 was initiated on June 24, 2024, to propose a new procedure: SOP PD289 - 00 Handling of Gloves in Manufacturing and Packing Area, Effective Date: October 03, 2024. However, the SOP does not include provisions for notifying the supervisor or documenting any potential future incidences concerning ripped pieces of gloves.
投诉 #PC/NCR/FDF/24 - 024 于 2024 年 5 月 10 日发起,涉及 ×× 毫克的 ×× 片剂,批号 ××。原因是客户在 2024 年 4 月 23 日打开密封瓶时,发现了医用级别的手套碎片,该碎片可能是手套的手指部分。经确定,手套可能在片剂检验前的 ×× 活动中撕裂,然后 ×× 片剂并装入(瓶中)。CAPA/030/24 于 2024 年 6 月 24 日启动,旨在提出一项新程序:SOP PD289 - 00《生产和包装区域手套的处理》,生效日期:2024 年 10 月 3 日。然而,该 SOP 没有包含通知主管或记录未来任何与手套撕裂碎片相关的潜在事件的条款。
OBSERVATION 3
观察项3
The responsibilities and procedures applicable to the quality control unit are not in writing and fully followed.
适用于质量控制部门的职责和程序未形成书面文件且未被完全遵守。
A) Complaint investigations that are involved with field alert reporting activities are not logged into the Field Alert Report Log (Annexure I) for further follow - up and reference as per SOP QA084 - 02, Field Alert Report, Effective Date: December 31, 2022, requirement. This include the lack of documentation in the Field Alert Report Log for Complaint MC 036/20, Complaint Received on: December 04, 2020 by the customer for ×× tablets ×× mg, Batch No. ×× Manufacturing, Date: 01/2020, Exp. Date: ×× Due to pharmacist stating that one completed seal bottle had ×× tablets under the strength of ×× mg and ×× tablet under the strength of ×× mg, Initial FAR Date: December 08, 2020, Final FAR Date: December 08, 2020. According to General Manager of Quality Assurance (QA), this event was not logged to procedural unawareness by the QA department.
涉及现场警戒报告活动的投诉调查未按照 SOP QA084 - 02《现场警戒报告》(生效日期:2022 年 12 月 31 日)的要求,记录到《现场警戒报告日志》(附件 I)中,以作进一步跟进和参考。这包括《现场警戒报告日志》中缺少对投诉 MC 036/20 的记录,该投诉于 2020 年 12 月 4 日由客户针对 ×× 毫克的 ×× 片剂(批号 ××,生产日期:2020 年 1 月,有效期:××)提出,原因是药剂师称一个完整密封的瓶子里有 ×× 片含量低于 ×× 毫克的片剂和 ×× 片含量低于 ×× 毫克的片剂。初始 FAR 日期:2020 年 12 月 8 日,最终 FAR 日期:2020 年 12 月 8 日。据质量保证(QA)总经理称,由于 QA 部门对程序不了解,该事件未被记录。
B) There is no instrument usage log for Analytical Balance #PD/AWB - 06 used to routinely perform in - process analytical testing of compressed tablets of various drug products manufactured in Production Block ×× In addition, the instrument usage log for friability test apparatus PD/FBL - 01 has not been completed as per SOP PD240 - 02 Operation, Cleaning and Calibration of Friability Test Apparatus, Effective Date: October 28, 2023, section 6.2 Annexure II. Usage Log for Friability Test Apparatus (PD240 - F02 - 01) requirement. According to General Manager of Quality Assurance (QA), this was due to procedural unawareness by operators and QA.
用于对生产区域 ×× 生产的各类药品压片进行常规过程分析测试的分析天平 #PD/AWB - 06 没有仪器使用日志。此外,脆碎度测试仪 PD/FBL - 01 的仪器使用日志未按照 SOP PD240 - 02《脆碎度测试仪的操作、清洁和校准》(生效日期:2023 年 10 月 28 日)第 6.2 节附件 II《脆碎度测试仪使用日志(PD240 - F02 - 01)》的要求完成。据质量保证(QA)总经理称,这是由于操作人员和 QA 对程序不了解。
C) There is no documented rationale and quality risk assessment for assigning the same User ID and passwords shared amongst production block operators, supervisors, and managers for login into manufacturing equipment Human Machine Interface (HMI). For example, ×× operators are provided with the supervisor level password for ×× Equipment ID: PD ×× 01 and ×× Equipment ID: PD ×× 02 as per SOP PD052 - 08 Operation of ×× (Capacity) ×× Effective Date: November 24, 2023. This is despite the software vendor manual stating that supervisor level password prevents access to the program details, and it should be known only to the supervisor or in charge of the plant. The supervisor level user access allows for edits of the following data points:
对于为生产区域的操作员、主管和经理分配相同的用户 ID 和密码以登录生产设备的人机界面(HMI),没有书面的基本原理和质量风险评估。例如,根据 SOP PD052 - 08《××(产能)×× 的操作》(生效日期:2023 年 11 月 24 日),×× 操作员被提供了用于 ×× 设备 ID:PD ×× 01 和 ×× 设备 ID:PD ×× 02 的主管级密码。尽管软件供应商手册中说明主管级密码可防止访问程序细节,且该密码应仅为主管或工厂负责人所知,但实际情况并非如此。主管级用户权限允许编辑以下数据点:
Process Time (total cycle time)
过程时间(总循环时间)
×× Off time (time during which ×× is switched off or during the time of ×× is taking place).
×× 关闭时间(×× 关闭期间或 ×× 发生期间的时间)。
On and Off (time duration during which ××)
开启和关闭(×× 期间的持续时间)
On and Off (time during which ××)
开启和关闭(×× 期间的时间)
set point
设定点
low and high limit
上下限
setpoint.
设定点。
The following additional manufacturing equipment Human Machine Interface (HMI) screens also have shared user IDs and passwords for Production Block ×× in which operators are assigned supervisor level access:
以下额外的生产设备人机界面(HMI)屏幕对于生产区域 ×× 也存在共享的用户 ID 和密码,在这些屏幕中,操作员被分配了主管级访问权限:
D) On August 06, 2025, I observed that the Packaging Supervisor was able to cut and delete logged software audit trail and packaging batch data files within the Countec Data System Windows application account used for the ×× Bottle Packing Line ×× operations. The account login is also shared between operators and packaging supervisors. According to the IT Administrator, this was an oversight.
2025 年 8 月 6 日,我观察到包装主管能够在用于 ×× 瓶装包装线 ×× 操作的 Countec 数据系统 Windows 应用程序账户内,剪切和删除已记录的软件审计追踪和包装批次数据文件。该账户登录信息也在操作员和包装主管之间共享。据 IT 管理员称,这是一个疏忽。
OBSERVATION 4
观察项4
Equipment and utensils are not cleaned, maintained and sanitized at appropriate intervals to prevent contamination that would alter the safety, identity, strength, quality or purity of the drug product.
设备和器具未在适当的时间间隔进行清洁、维护和消毒,以防止污染,而这种污染可能会改变药品的安全性、鉴别性、效价、质量或纯度。
A) During the inspectional walkthrough on August 4th, 2025, visible ×× stains were observed within the ×× which remains open during ×× operations for drug products intended for the U.S and other global markets. PD ×× 01 was last used to manufacture ×× tablets ×× mg, ×× mg, and ×× mg, Batch No. ×× prior to Product Changeover (Type-B) cleaning being completed on July 30, 2025. However, the following test results were reported after swab sampling performed as per the current inspection request for the same equipment on August 4th, 2025:
在 2025 年 8 月 4 日的检查巡视中,在 ×× 内观察到可见的 ×× 污渍,该 ×× 在针对美国和其他全球市场的药品的 ×× 操作期间一直处于开启状态。PD ×× 01 最后一次用于生产 ×× 毫克、×× 毫克和 ×× 毫克的 ×× 片剂,批号 ××,之后在 2025 年 7 月 30 日完成了产品转换(B 型)清洁。然而,根据本次检查要求,于 2025 年 8 月 4 日对同一设备进行擦拭取样后,报告了以下测试结果:
An additional peak in the sample chromatograms for all ×× swab sample locations was identified to be traces of ×× which is a ×× used in the treatment of ×× The identified peak for ×× had a higher area response compared to previously last manufactured product: ×× For example:
在所有 ×× 擦拭取样位置的样品色谱图中,发现了一个额外的峰,经鉴定为 ×× 的痕迹,×× 是用于治疗 ×× 的一种 ××。与之前最后生产的产品 ×× 相比,×× 的已鉴定峰具有更高的面积响应。例如:
Swab Analysis results for ×× Duct
×× 管道的擦拭分析结果
×× Tablets ×× mg, was last manufactured on March 2025 using PD ×× 01. ×× additional drug product batches have been manufactured using the same equipment since then for the U.S and other global markets, including:
×× 毫克的 ×× 片剂最后一次是在 2025 年 3 月使用 PD ×× 01 生产的。从那以后,使用同一设备为美国和其他全球市场生产了 ×× 批额外的药品,包括:
×× Tablets ×× mg and ×× mg (USA), Batch No.
×××× 毫克和 ×× 毫克的 ×× 片剂(美国),批号 ××
Mfg. Date: ×× Status: Dispatched
生产日期:×× 状态:已发运
×× Tablets ×× mg and ×× mg (USA), Batch No.
×××× 毫克和 ×× 毫克的 ×× 片剂(美国),批号 ××
Mfg. Date: ×× Status: Dispatched
生产日期:×× 状态:已发运
×× Tablets ×× mg and ×× mg (USA), Batch No.
×××× 毫克和 ×× 毫克的 ×× 片剂(美国),批号 ××
Mfg. Date: ×× Status: Dispatched
生产日期:×× 状态:已发运
×× Tablets ×× mg and ×× mg (USA), Batch No.
×××× 毫克和 ×× 毫克的 ×× 片剂(美国),批号 ××
Mfg. Date: ×× Status: Dispatched
生产日期:×× 状态:已发运
This is despite the following product changeover cleaning steps being sign - off as completed and verified after production of ×× Batch No.
××尽管在 ×× 批号 ×× 的产品生产后,以下产品转换清洁步骤已被签署为完成并经过验证,但仍出现了上述情况。
B) During the inspectional walkthrough on August 4th, 2025, visible ×× product residue was observed on the ×× upper and outer surfaces, corner surface of the empty capsule reservoir, and outer surface of the empty capsules reservoir within Capsule filling machine (ID: PD/CFM - 03). PD/CFM - 03 was last used to manufacture ×× capsules USP ×× mg, Batch No. ×× on July 22, 2025, prior to Product Changeover (Type B) cleaning being completed on July 24, 2025. However, the following test results were reported after swab sampling performed as per the current inspection request for the same equipment on August 4th, 2025:
在 2025 年 8 月 4 日的检查巡视中,在胶囊填充机(ID:PD/CFM - 03)内的 ×× 上表面和外表面、空胶囊储槽的角表面以及空胶囊储槽的外表面观察到可见的 ×× 产品残留。PD/CFM - 03 最后一次用于生产 ×× 毫克的USP胶囊,批号 ××,时间为 2025 年 7 月 22 日,之后在 2025 年 7 月 24 日完成了产品转换(B 型)清洁。然而,根据本次检查要求,于 2025 年 8 月 4 日对同一设备进行擦拭取样后,报告了以下测试结果:
This is despite the following product changeover cleaning steps being sign - off as completed and verified:
尽管以下产品转换清洁步骤已被签署为完成并经过验证,但仍出现了上述情况:
C) On August 08, 2025, the following poor equipment conditions were observed for Compression Machine PD/TCM - 03 located in Production Block ×× and routinely used to compressed tablets for the U.S market:
2025 年 8 月 8 日,对于位于生产区域 ×× 且常规用于为美国市场生产压片的压片机 PD/TCM - 03,观察到以下不良设备状况:
×× stains and spots observed on the ×× and
××在 ×× 和 ×× 上观察到 ×× 污渍和斑点
Visible dents on the ×× of the
××在 ×× 的 ×× 上有可见的凹痕
Damaged/chipping parts for the ×× In addition, ×× was observed across the
×××× 的部件有损坏 / 碎裂情况。此外,在 ×× 上观察到 ××
A shiny metal particle found on the ×× of the compression machine.
在压片机的 ×× 上发现了一个有光泽的金属颗粒。
The equipment was last to manufacture ×× Tablets USP ×× mg, Batch No. ×× intended for the U.S market.
该设备最后一次用于生产 ×× 毫克的USP片剂,批号 ××,用于美国市场。
D) During my inspection walkthrough on August 04, 2025, I observed what appeared to be very visible inner stress cracks and scratches within the product - contact view glass of ×× PD ×× 01. PD ×× 01 is routinely used for ×× operations for drug products distributed to the U.S and other global markets, including: ×× Tablets ×× and ×× mg last manufactured on May 9th, 2025. There has been no incident report raised or maintenance check conducted for this matter until the current inspection.
在 2025 年 8 月 4 日的检查巡视中,我观察到 ×× PD ×× 01 与产品接触的视镜玻璃内似乎有非常明显的内应力裂纹和划痕。PD ×× 01 常规用于向美国和其他全球市场分销的药品的 ×× 操作,包括:最后一次生产于 2025 年 5 月 9 日的 ×× 毫克和 ×× 毫克的 ×× 片剂。在本次检查之前,没有就此事提交过事故报告或进行过维护检查。
E) During my inspection walkthrough on August 06, 2025, two visible large cracks was observed within the ×× directly above the tablet and capsule ×× of Tablets/Capsule Counting Machine ID: PD/TCC - 03. According to the Packaging Supervisor, the cracks had recently occurred. However, there is no documentation of this incident, timeline of the occurrence, or maintenance check conducted for this matter until the current inspection.
在 2025 年 8 月 6 日的检查巡视中,在片剂 / 胶囊计数机(ID:PD/TCC - 03)的片剂和胶囊 ×× 正上方的 ×× 内观察到两条明显的大裂缝。据包装主管称,这些裂缝是最近出现的。然而,在本次检查之前,没有关于该事件的文件记录、发生时间线或就此事进行的维护检查。
OBSERVATION 5
观察项5
Written procedures are not established for the cleaning and maintenance of equipment, including utensils, used in the manufacture, processing, packing or holding of a drug product.对于用于药品生产、加工、包装或贮存的设备(包括器具)的清洁和维护,未制定书面程序。
A) Your firm's cleaning validation revised Protocol No. ECV/NP/008-05, MACO Establishment Protocol, Effective Date: January 25, 2019, established (Total Score: ××) as the worst-case product to conduct cleaning validation study for all non-dedicated equipment product contact surfaces used to product U.S and non-U.S marketed finished drug products. This was based on a total score and assessment done for toxicity, solubility and hardest to clean considerations. However, there is no documented scientific justification as to why ×× tablets (Total Score: ××) was instead selected to perform the cleaning validation study and 2023 recleaning validation study for all equipment product contact surfaces, given the following total scores reported in the protocol as per the table below:
贵公司的清洁验证修订方案编号 ECV/NP/008-05《MACO 建立方案》,生效日期:2019 年 1 月 25 日,确定(总得分:××)为最差情况产品,用于对所有用于生产美国和非美国市场成品药的非专用设备产品接触表面开展清洁验证研究。这是基于对毒性、溶解性和最难清洁情况的总得分和评估。然而,鉴于方案中如下表报告的总得分,对于为何选择 ×× 片剂(总得分:××)来对所有设备产品接触表面开展清洁验证研究和 2023 年再清洁验证研究,没有书面的科学依据:
Total score based on Toxicity, Solubility, and Hardest to clean considerations:
基于毒性、溶解性和最难清洁情况的总得分:
During the inspection, the Assistant General Manager of Quality Assurance (QA) stated that ×× tablets, Total Score: ×× was mistakenly chosen instead of the actual previously identified hardest-to-clean product ×× (Total Score: ××) to conduct the cleaning validation study due to ×× having the lowest maximum carry-over (MACO) limit.
在检查过程中,质量保证(QA)总经理助理表示,×× 片剂(总得分:××)被错误地选择,而非之前确定的实际最难清洁产品 ××(总得分:××)来开展清洁验证研究,原因是 ×× 具有最低的最大残留(MACO)限值。
B) Since the 2018 cleaning validation, there has been no further evaluation and cleaning validation study for more than ×× newly introduced finished drug products to assess overall toxicity, Solubility, and Hardest to clean considerations for shared non-dedicated equipment surfaces, including: ×× In addition, your firm has performed a single cleaning verification study for certain finished drug products dating back to 2015, without any written justification on the frequency, timeline, drug product selection, and selection of only some hardest-to-clean areas for swab analysis versus all hardest-to-clean areas reviewed during visual inspection.
自 2018 年清洁验证以来,对于超过 ×× 种新推出的成品药,没有进一步的评估和清洁验证研究,以评估共享非专用设备表面的整体毒性、溶解性和最难清洁情况,包括:×× 此外,贵公司自 2015 年以来对某些成品药仅开展了一次清洁验证研究,对于擦拭分析仅选择部分最难清洁区域而非目视检查中审查的所有最难清洁区域,以及频率、时间线、药品选择,均没有书面依据。
C) There is no documented scientific rationale and quality risk assessment for having one cleaning validation study representative for all finished drug product profiles. The 2018 cleaning validation study protocol and report did not assess the differences in physical properties and manufacturing processes for various types of drug products, including: capsules ×× and compressed tablets ×× Tablets was instead selected as the sole drug product to conduct the cleaning validation study.
对于用一项清洁验证研究代表所有成品药情况,没有书面的科学依据和质量风险评估。2018 年清洁验证研究方案和报告未评估各类药品在物理性质和生产工艺上的差异,包括:胶囊 ×× 和压片 ××。反而选择 ×× 片剂作为开展清洁验证研究的唯一药品。
D) SOP PD291-01, Operation and Cleaning of Tablet Compression Machine, Effective Date: March 13, 2025, instructs operators to solely use ×× and a ×× cloth for cleaning surfaces of the ×× and table compression machine surface. However, ×× was not assessed as part of the original cleaning validation study and sampling plan. Only ×× was evaluated for ×× residue for all surfaces. According to the Production Manager, the firm has decided to use ×× as part of their routine cleaning operations instead of ×× to prevent rusting of equipment surfaces. He stated the ×× was not evaluated as part of the cleaning validation program due to an oversight.
SOP PD291-01《压片机的操作与清洁》,生效日期:2025 年 3 月 13 日,指导操作人员仅使用 ×× 和一块 ×× 布来清洁 ×× 和压片机表面。然而,×× 未作为原始清洁验证研究和取样计划的一部分进行评估。仅对所有表面的 ×× 残留进行了 ×× 评估。据生产经理称,公司已决定将 ×× 作为日常清洁操作的一部分,而非 ××,以防止设备表面生锈。他表示,由于疏忽,×× 未作为清洁验证程序的一部分进行评估。
E) During my inspection walkthrough on August 04, 2025, I observed an unidentified ×× bowl stored within Module ×× area, Production Block ×× last used to manufacture ×× tablets ×× mg, and ×× mg, Batch No. ×× According to the Production Manager, the ×× bowl is not used in the production of ×× and is instead used as part of the ×× Equipment ID: PD ×× -04. Equipment ID: PD ×× -04 was last documented as being used in the ×× area for manufacturing operations involving ×× Tablets, USP ×× mg, ×× mg, and ×× mg, Batch No. ×× on July 27, 2025. However, the Production Manager and Senior General Manager of Operations, was unable to explain and provided documentation for why, how, and when the ×× was placed in the different drug production area for a different product.
在 2025 年 8 月 4 日的检查巡视中,我观察到在生产区域 ×× 的模块 ×× 区域内存放着一个不明 ×× 碗,该区域最后一次用于生产 ×× 毫克和 ×× 毫克的 ×× 片剂,批号 ××。据生产经理称,该 ×× 碗不用于 ×× 的生产,而是作为 ××(设备 ID:PD ×× -04)的一部分使用。设备 ID:PD ×× -04 最后一次有记录的使用是在 2025 年 7 月 27 日,在 ×× 区域用于涉及 ×× 毫克、×× 毫克和 ×× 毫克的USP ×× 片剂(批号 ××)的生产操作。然而,生产经理和运营高级总经理无法解释并提供文件说明为何、如何以及何时将 ×× 放置在不同药品生产区域用于不同产品。
F) On August 06, 2025, ×× (ID: PD ×× 1) was observed to be unplugged/disconnected and stationed on the production floor with other equipment parts. According to General Manager (GM) of QA, the ×× is disconnected after batch production involving ×× tablets USP ×× mg, ×× mg, and ×× mg. ID: PD ×× was last used on June 12, 2025, for Batch No. ×× It was also previously used on May 31, 2025, to produce Batch No. ×× The batch record instructions require the operator to maintain the ×× at a ×× between ×× However, there is no procedure to verify the functionality of the ×× after reconnection and before usage for batch production operations.
2025 年 8 月 6 日,观察到 ××(ID:PD ×× 1)处于未插电 / 断开连接状态,并与其他设备部件一起放置在生产车间。据 QA 总经理称,在涉及 ×× 毫克、×× 毫克和 ×× 毫克的USP ×× 片剂的批次生产后,×× 会被断开连接。ID:PD ×× 最后一次使用是在 2025 年 6 月 12 日,用于批号 ×× 的生产。它之前还在 2025 年 5 月 31 日用于生产批号 ×× 的产品。批次记录说明要求操作人员将 ×× 保持在 ×× 至 ×× 之间。然而,没有程序来验证 ×× 在重新连接后以及用于批次生产操作之前的功能。
OBSERVATION 6
观察项6
The accuracy, sensitivity, specificity and reproducibility of test methods have not been established.
检测方法的准确性、灵敏度、特异性和可重复性尚未得到确认。
A) The ×× analytical method for ×× Tablets USP ×× mg, ×× mg, ×× mg, ×× mg, and ×× mg, which is routinely used in - process testing, has not been evaluated for accuracy, sensitivity, specificity and reproducibility according to the Assistant General Manager of QA. This includes that lack of evaluation for the ×× sample preparation procedure and suitability of sample mass differences. For example:
据质量保证(QA)总经理助理称,用于 ×× 毫克、×× 毫克、×× 毫克、×× 毫克和 ×× 毫克的USP ×× 片剂的 ×× 分析方法,该方法常规用于过程中检测,但尚未对其准确性、灵敏度、特异性和可重复性进行评估。这包括对 ×× 样品制备程序以及样品质量差异的适用性缺乏评估。例如:
×× testing protocol for ×× Tablets USP ×× mg, ×× mg, ×× mg and ×× mg /In - process instructs Quality Assurance (QA) personnel to collected of the finished product individual dosage form. For ×× Tablets USP ×× mg, ×× Batch No. ×× this equates to a sample size quantity specification of: ×× mg to ×× mg according to the range of the sample size allowed to be taken by QA for establishing the lower and higher Assist QA Manager, there is no study available for ×× testing. In addition, the following out - of - specification (OOS) results were reported since June 2021 and were attributed to probable sampling errors:
用于 ×× 毫克、×× 毫克、×× 毫克和 ×× 毫克的USP ×× 片剂 / 过程中的 ×× 检测方案,指导质量保证(QA)人员收集成品的单个剂型。对于批号为 ×× 的 ×× 毫克的USP ×× 片剂,根据 QA 为确定上下限可采集的样品量范围,这相当于样品量规格为:×× 毫克至 ×× 毫克。据助理 QA 经理称,没有可用于 ×× 检测的研究。此外,自 2021 年 6 月以来报告了以下超出规格(OOS)的结果,这些结果被归因于可能的取样误差: