4.26 Cleanroom classification is part of the cleanroom qualification and is a method of assessing the level of air cleanliness against a specification for a cleanroom or clean air equipment by measuring the total particle concentration. Classification activities should be scheduled and performed in order to avoid any impact on process or product quality. For example, initial classification should be performed during simulated operations and reclassification performed during simulated operations or during aseptic process simulation (APS).
洁净室分级是洁净室确认的一部分,是一种根据洁净室或洁净空气设备的标准通过测定总微粒浓度来评估空气洁净度水平的方法。分级活动的安排和执行,应避免对工艺或产品质量产生任何影响。例如,应在模拟操作期间进行初步分级,在模拟操作或无菌工艺模拟试验(APS)期间进行再分级。
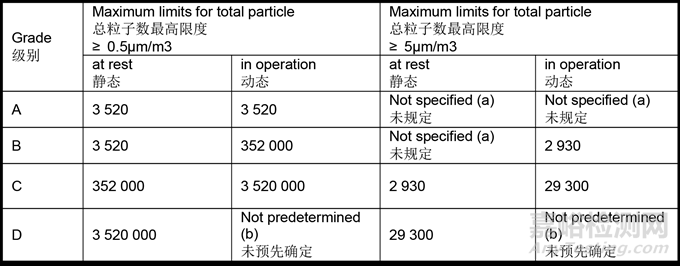
4.27 For cleanroom classification, the total of particles equal to or greater than 0.5 and 5 μm should be measured. This measurement should be performed both at rest and in simulated operations in accordance with the limits specified in Table 1.
对于洁净室分级,应测定等于或大于0.5μm和5μm的微粒总数。应按照表1中规定的限度,在静态和模拟操作中进行测定。
Table 1: Maximum permitted total particle concentration for classification
表1:各级别允许的最大总微粒浓度
(a) Classification including 5μm particles may be considered where indicated by the CCS or historical trends.
在CCS或历史趋势中有说明的情况下,可以考虑包括5μm微粒的分级。
(b) For grade D, in operation limits are not predetermined. The manufacturer should establish in operation limits based on a risk assessment and routine data where applicable.
对于D级区,没有预先确定的动态限度。生产商应根据风险评估和适用的常规数据建立动态限度。
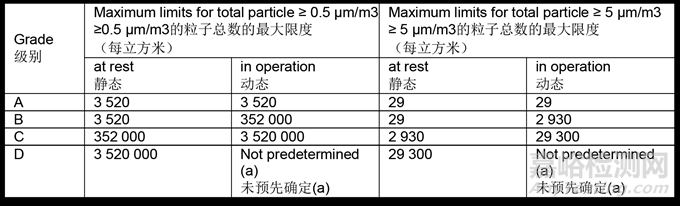
9.14 A total particle monitoring program should be established to obtain data for assessing potential contamination risks and to ensure the maintenance of the environment for sterile operations in a qualified state.
应建立总粒子数监测计划,将获得的数据用于评估潜在污染风险,并确保无菌操作环境维持在经确认的状态。
9.15 The limits for environmental monitoring of airborne particle concentration for each graded area are given in Table 5.
各级别空气悬浮颗粒浓度环境监测限度见表5。
Table 5: Maximum permitted total particle concentration for monitoring.
表5:监测最大允许的总粒子浓度。
(a) For grade D, in operation limits are not predetermined. The manufacturer should establish in operation limits based on a risk assessment and on routine data, where applicable.
对于D级,动态限度未预先确定。生产商应根据风险评估和适用的常规数据建立动态限度。
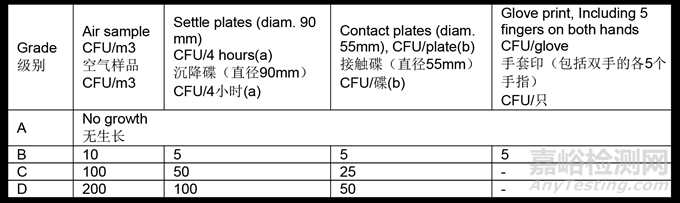
4.31 The microbial contamination level of the cleanrooms should be determined as part of the cleanroom qualification. The number of sampling locations should be based on a documented risk assessment and the results obtained from room classification, air visualization studies and knowledge of the process and operations to be performed in the area. The maximum limits for microbial contamination during qualification for each grade are given in Table 2. Qualification should include both “at rest” and “in operation” states.
洁净室的微生物污染水平应作为洁净室确认的一部分进行确定。采样点的数量应基于书面的风险评估、房间分级结果、气流可视化研究以及对该区域要进行的工艺和操作的了解。表2给出了各级别确认过程中的微生物污染最大限度。确认包含“静态”和“动态”条件中。
Table 2: Maximum permitted microbial contamination level during qualification
表2:确认过程中允许的最大微生物污染水平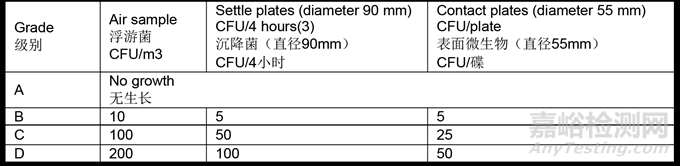
(a) Settle plates should be exposed for the duration of operations and changed as required after a maximum of 4 hours. Exposure time should be based on recovery studies and should not allow desiccation of the media used.
沉降碟应在操作期间暴露,并在最多4小时后按需要更换。暴露时间应基于回收率研究,并且避免所所用培养基干燥。
9.30 Action limits for viable particle contamination are shown in Table 6
表列出了活性粒子污染的行动限
(a) Settle plates should be exposed in grade A and B areas for the duration of operations (including equipment set-up) and changed as required after a maximum of 4 hours (exposure time should be based on validation including recovery studies and it should not have any negative effect on the suitability of the media used).
沉降碟应在操作期间(包括设备安装)暴露在A级和B级区,并在最多4小时后按要求更换(暴露时间应基于验证,包括回收研究,不应对所用培养基的适用性产生任何负面影响)。
- For grade C and D areas, exposure time (with a maximum of 4 hours) and frequency should be based on QRM.
-对于C级和D级区,暴露时间(最多4小时)和频率应基于质量风险管理(QRM)。
- Individual settle plates may be exposed for less than 4 hours.
-单个沉降碟的暴露时间可以少于4小时。
(b) Contact plate limits apply to equipment, room and gown surfaces within the grade A and grade B areas. Routine gown monitoring is not normally required for grade C and D areas, depending on their function.
接触碟限度适用于A级和B级区内的设备、房间和洁净服表面。C级和D级区通常不需要进行常规工作服监测,具体取决于该区域的功能。
(c) It should be noted that for grade A, any growth should result in an investigation.
应注意,A级区域如有任何长菌情况都应进行调查。