您当前的位置:检测资讯 > 法规标准
嘉峪检测网 2023-09-04 08:41
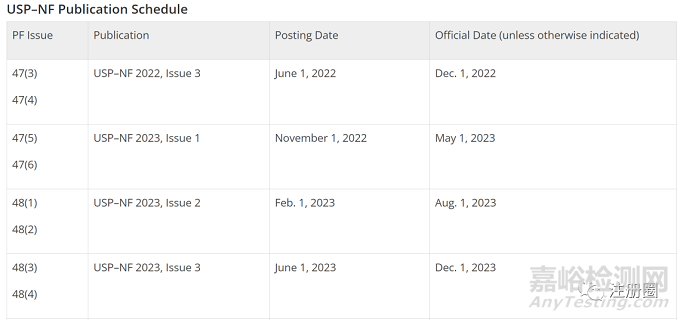
| USP各论示例 |
 |
| 修订类型 | Erratum/Errata(勘误) |
| 修订符号和文本标注 |
 (ERR 1-Feb-2019) (ERR 1-Feb-2019) |
| USP官方释义1 | Errata are Accelerated Revisions representing corrections to items erroneously published. |
| 译文:勘误是按加速修订程序,对错误发布的项目进行的更正。 | |
| USP官方释义2 | Errata published in the errata table are errors, clarifications, or missing information such as inaccuracies in chemical formulas, mathematical equations relevant to analyses and/or inaccurate descriptions of the apparatus, or materials used to perform analytical tests. Typically Errata are changes that do not have a broad impact on the standard. Errata are not subject to public notice and comment. |
| 译文:勘误表中公布的勘误是错误、澄清或遗漏的信息,如与分析有关的化学公式、数学公式不准确和/或对用于进行分析测试的仪器或材料的描述不准确。勘误表通常是对标准没有广泛影响的更改。勘误表无需公示和征求意见。 | |
| USP官方释义3 | Errata refer to an accelerated revision vehicle used to correct published content in a USP compendium that does not accurately reflect the intended requirements of a standard as approved by the responsible Expert Committee. Errata published on the USP webpage most often become official/effective on the first day of the month following publication; however, in some cases the official date of the Errata may be extended to align with previously published revisions that have not yet become official. The official or effective date will be noted in the revised version of these standards as well as in the revision tagging associated with the specific Errata. |
| 译文:勘误指的是一种加速修订工具,用于纠正USP中未准确反映主管专家委员会批准的标准预期要求的已出版内容。USP网页上公布的勘误表通常在公布后当月的第一天正式生效;但在某些情况下,勘误的正式生效日期可能会延长,以便与之前公布的尚未正式生效的修订版保持一致。正式或生效日期将在这些标准的修订版以及与具体勘误相关的修订标记中注明。 | |
| 勘误表公布网址 | https://www.uspnf.com/official-text/errata-table |
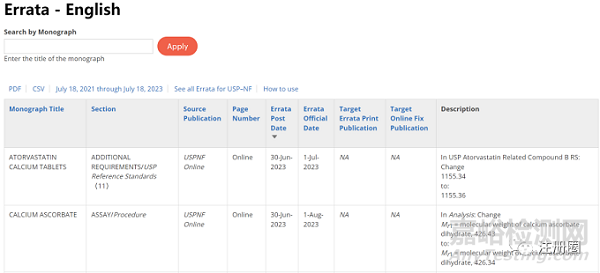 |
|
| 注:可在搜索框中输入关键词进行检索。 |
| USP各论示例 |
 |
| 修订类型 | Interim Revision Announcement(临时修订声明) |
| 修订符号和文本标注 |
|
| USP官方释义1 | IRAs are an expedited mechanism for making revisions official. An IRA appears in PF first as a Proposed Interim Revision Announcement with a 90-day comment period. If there are no significant adverse comments, the IRA becomes official and is immediately incorporated into the USP-NF with the official date indicated. |
| 译文:临时修订声明是一种使修订生效的快速机制。IRA首先作为“拟议临时修订声明”出现发布在PF上,有90天的征求意见期。如果没有重大的反对意见,IRA将在公示结束后立即成为正式版本,并立即纳入USP-NF并注明正式日期。 | |
| USP官方释义2 | IRAs are used: (1) to correct substantive errors (beyond the scope of Errata); (2) to address non-urgent patient safety or compliance issues where public comment on the revision is warranted; (3) to address time-sensitive reference standard (RS) issues, including but not limited to changes in a form or a presentation of an RS; and (4) to correct non-working tests in a standard. |
| 译文:IRA用于:(1) 纠正实质性错误(超出勘误表范围);(2) 解决非紧急的患者安全或合规性问题,在此情况下,公众有必要对修订提出意见;(3) 解决具有时效性的标准品/对照品(RS)问题,包括但不限于更改RS的形式或表述方式;(4) 纠正标准中的非工作检测。 | |
| IRA正式文本发布网址 |
https://www.uspnf.com/official-text/iras
|
| USP各论示例 |
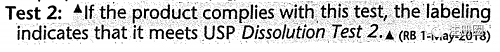 |
| 修订类型 | Revision Bulletin(修订公告) |
| 修订符号和文本标注 |
 (RB 1-May-2018) (RB 1-May-2018) |
| USP官方释义1 | Revision Bulletins are Accelerated Revisions to official text or postponements that require expedited publication. They generally are official immediately unless otherwise specified in the Revision Bulletin. |
| 译文:修订公告是对正式文本的加速修订或需要快速发布的延期公告。除非《修订公告》中另有说明,否则它们通常立即成为正式文本。 | |
| USP官方释义2 | Revision Bulletins are used to address issues that require rapid publication of official text, namely: (1) to correct substantive errors (beyond the scope of Errata); (2) to address urgent patient safety issues; (3) to address compliance issues where public comment is not warranted; (4) to issue postponements in response to Requests for Postponement (see Rules); (5) to remove postponed text from the official text of the compendium when the postponement has not been resolved within the normal revision lifecycle; and (6) to effectuate appeals-related revisions (see Rules). |
| 译文:修订公告用于解决需要快速发布正式文本的问题,包括:(1) 纠正实质性错误(超出勘误表范围);(2) 解决紧急的患者安全问题;(3) 解决无需征求公众意见的合规性问题;(4) 根据延期请求发布延期公告;(5) 当延期问题未能在正常修订周期内解决时,从药典正式文本中删除延期文本;(6) 实施与申诉有关的修订。 | |
| USP官方释义3 | Revision Bulletins are most often official on the first day of the month that follows the month in which they are published. However, in some cases the official date of the Revision Bulletin may be extended to align with previously published revisions that have not yet become official. The official date will be noted in the revised version of these standards as well as in the revision tagging associated with the specific Revision Bulletin. |
| 译文:修订公告通常在发布当月的下一个月第一天正式生效。不过,在某些情况下,修订公告的正式日期可能会延长,以便与之前发布但尚未正式生效的修订版本保持一致。正式日期将在这些标准的修订版本以及与特定修订公告相关的修订标记中注明。 | |
| RB正式文本发布网址 |
https://www.uspnf.com/off icial-text/revision-bulletins icial-text/revision-bulletins |
| USP各论示例1 |
 |
| USP各论示例2 |
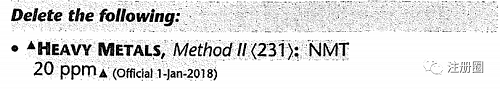 |
| 修订类型 | Compendial Notice(药典通知) |
| • Chapter Reference(通则引用) | |
| 修订符号和文本标注 |
 (CN 1-May-2020) (CN 1-May-2020) |
 (Official 1-Jan-2018) (Official 1-Jan-2018) |
|
| USP官方释义1 | Compendial Notice revisions are used sparingly to update, edit, or correct issues in compendial text that do not fit any of the categories described above, but where expeditious revision serves public health and quality needs and where public comment is not warranted. |
| 译文:药典通知修订很少用于更新、编辑或纠正药典文本中不符合上述任何类别的问题,但适用于快速修订可满足公众健康和质量需求且无需征求公众意见的情况。 | |
| USP官方释义2 | Reference Changes represent an example of changes made via Compendial Notice. Sometimes it is necessary for USP to modify general chapter titles, section titles, appendix titles, reference to external resources, or similar text that may be referenced in standards throughout the USP–NF. Any reference changes that will have an impact on the interpretation or requirements of the standard are made using the routine revision process including publication for comment in PF. In cases in which an update appears to present no significant change in the affected standard, they are implemented through a direct update of the reference in that standard without providing an opportunity for notice and comment. In these cases, USP will publish on its webpage a Compendial Notice (CN) indicating the source change and any resulting references. Updates made through direct publication in the USP–NF will be clearly identified by symbols and shading. |
| 译文:参考或引用更改是一种通过药典通知进行的变更。有时,USP有必要修改通则大标题、章节标题、附录标题、外部资源引用或可能在USP-NF各标准中引用的类似文本。任何会对标准的解释或要求产生影响的参考或引用变更,都是通过常规修订程序进行的,包括在PF中公布征求意见。如果更新似乎不会对受影响的标准造成显著改变,则通过直接更新该标准中的参考或引用来实施,而不再通知和征求意见。在这些情况下,USP将在其网页上发布药典通知 (CN),说明来源更改和任何由此产生的参考或引用。在USP-NF中直接发布的更新将通过符号和阴影清楚地标识。 | |
| USP官方释义3 | Because some updates to references may be assigned delayed official dates to ensure that all related revisions become official together, the revision markup used to identify the reference updates will be dependent upon when the change will become official. Updates becoming official at the same time as the USP–NF vehicle in which they are published will be identified with the following revision markup, where CN denotes Compendial Notice: |
| • Changed text ▲ (CN 1-May-2019) | |
| Updates becoming official/effective with a greater than 6-month implementation period will be identified with the following revision markup: | |
| • Changed text ▲ (Official 1-May-2019) | |
| 译文:由于某些参考或引用来源的更新可能会被指定延迟的正式日期,以确保所有相关修订都能一起成为正式版本,因此用于标识参考资料更新的修订标记将取决于更改何时成为正式版本。 | |
| 与USP-NF版本同时成为正式版本的更新将使用以下修订标记来标识,其中CN表示药典通知: | |
| • 更改后的文本 ▲ (CN 1-May-2019) | |
| 正式生效/实施期超过6个月的更新将使用以下修订标记进行标识: | |
| • 更改后的文本 ▲ (Official 1-May-2019) | |
| 药典通知更新网址 |
https://www.uspnf.com/notices/new
|
| USP各论示例 |
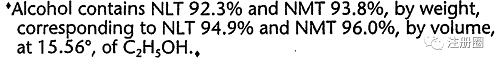 |
| 修订类型 | Harmonization(协调) |
| 修订符号和文本标注 |
 |
| USP官方释义1 | USP harmonizes pharmacopeial excipient monographs and general chapters through the Pharmacopeial Discussion Group (PDG), which includes representatives from the European, Japanese, and United States pharmacopeias and WHO (as an observer). According to the PDG definition, "a pharmacopeial general chapter or other pharmacopeial document is harmonized when a pharmaceutical substance or product tested by the document's harmonized procedure yields the same results and the same accept/reject decision is reached." |
| 译文:USP通过药典讨论组 (PDG) 协调药典辅料各论和通则,该讨论组包括来自欧洲药典、日本药典和美国药典以及WHO(作为观察员)的代表。根据药典讨论组的定义,“当药用物质或产品通过该文件的协调程序测试得出相同的结果,并做出相同的接受/拒绝决定时,该药典通则或其他药典文件即被协调”。 | |
| USP官方释义2 | The specific stages of the PDG Procedure (Process) involved in Harmonization are: |
| Pre-PDG | |
| Stage 1: Preparation of first draft | |
| Stage 2: Official Inquiry | |
| Stage 3: Consensus | |
| Stage 4: Regional adoption and implementation | |
| Stage 5. Inter-regional acceptance (for chapters previously evaluated by ICH Q4B for Regulatory Interchangeability) | |
| 译文:协调工作所涉及的 PDG 程序(过程)的具体阶段包括 | |
| PDG 前期 | |
| 第 1 阶段:编写初稿 | |
| 第 2 阶段:正式调研(征求意见) | |
| 第 3 阶段:协商一致 | |
| 第 4 阶段:地区通过和实施 | |
| 第 5 阶段:区域间接受(适用于之前经 ICH Q4B 评估为监管互换性的章节) | |
| 阶段4通知公布网址 |
https://www.usp.org/harmonization-standards/pdg/stage-4-notices
|
| Revision Type | Revision Symbol and Text |
| 修订类型 | 修订符号和文本 |
|
In Process Revision (Book or Supplement) 正在修订(正式或增补) |
|
| Interim Revision Announcement | (IRA 1-Jul-2020) |
| 临时修订声明 | |
| Revision Bulletin |
|
| 修订公告 | |
| Harmonization |
|
| 协调(ICH) | |
| Errata |
|
| 勘误 | |
| Chapter References |
|
| 通则引用 | (Official 1-Jul-2020) |
| Pending |
|
| 待定 |

来源:注册圈


