您当前的位置:检测资讯 > 科研开发
嘉峪检测网 2022-03-05 06:59
目前,氢气被证明具有肿瘤抑制的效果,但是存在治疗效率低或体内生物安全性等问题。本研究使用镁基生物材料在肿瘤环境中的降解,设计了一种可控的局部氢气释放方法,具有高效的肿瘤抑制效果和良好的生物相容性,进一步依据mRNA转录组测序结果揭示氢分子诱导肿瘤凋亡的具体作用机制。
01、研究内容简介
近十年来,氢分子被发现具有疾病治疗效果,如炎症、脑损伤、阿尔兹海默症等。目前,氢分子医学被广泛接受的作用机制是降低了具有毒性的活性氧的水平,如过氧亚硝基、羟自由基等。但是,氢分子针对特定疾病(如肿瘤)的完整作用机制通路仍不明确。
传统氢气由机体摄取的方式包括呼吸道、消化道、直接注射。这些氢气摄取方法对体内深部肿瘤的治疗效果有限,因为氢气具有高扩散性、低溶解性等特点,无法在肿瘤周围实现较高的溶解氢气浓度。为了解决这些问题,局部氢气释放的治疗策略被相继开发,如可以释放氢气的纳米颗粒直接注射或靶向到肿瘤组织,实现肿瘤周围较高的氢气富集,提高治疗效果。然而,这些具有不可降解性和细胞毒性的纳米颗粒也增加了患者健康风险。
作为可降解金属,镁具有相对较低的电极电位可以与人体内的体液反应生成氢氧化镁并释放氢气。同时,大量前期结果证明,镁金属植入物在动物或人体内具有生物安全性和良好的生物相容性。生物镁金属材料在体内可以创造一个高饱和氢气环境,在镁植入物周围氢气浓度可达到1.46 mM,高于大气压下水中饱和氢气浓度0.6 mM。尽管镁金属作为氢气载体在肿瘤疾病中治疗效果已被报道,但是氢分子如何诱导肿瘤细胞凋亡和相关作用通路仍需要进一步探究。
在本研究中,通过改变肿瘤弱酸性微环境和镁金属暴露在肿瘤周围的表面积,实现有效调控镁植入物的氢气释放速率的目的,通过使用不同浓度的氢气处理结直肠癌细胞,探索其诱导肿瘤细胞凋亡的氢气浓度范围,并在体内荷瘤小鼠模型中进一步验证。此外,本研究还通过对空白对照组和氢气处理组的细胞进行mRNA转录组测序,对比分析镁释放氢气抑制结直肠肿瘤的潜在机制。
实验结果表明,在肿瘤组织周围酸性环境中通过调整镁植入物表面积,可以在体液中控制氢气的释放速度,从而调节局部的氢气溶解浓度,实现局部可控的高效氢气输送方式 (Fig. 1)。
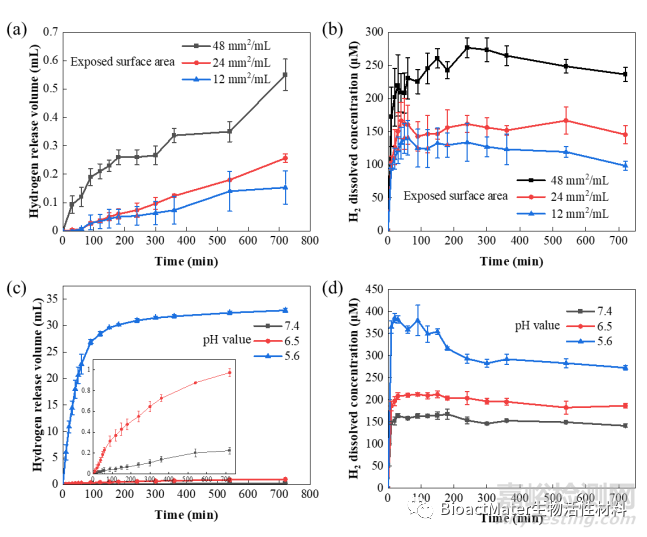
Fig.1 Controlled release kinetics of hydrogen from Mg. (a) The volume of released H2 and (b) the concentration of dissolved H2 in PBS solution increased with the exposed surface area (mm2 per mL PBS) of Mg plates (PBS: pH = 7.4); (c) the volume of released H2 and (d) the concentration of dissolved H2 in PBS increased as the pH value decreases.
使用氢气释放装置单独研究了镁的降解产物氢气对人结肠癌细胞HCT116的影响。通过活细胞成像系统和细胞学实验,发现氢气可以阻滞HCT116细胞分裂,诱导凋亡,最终抑制肿瘤细胞增殖 (Fig. 2)。
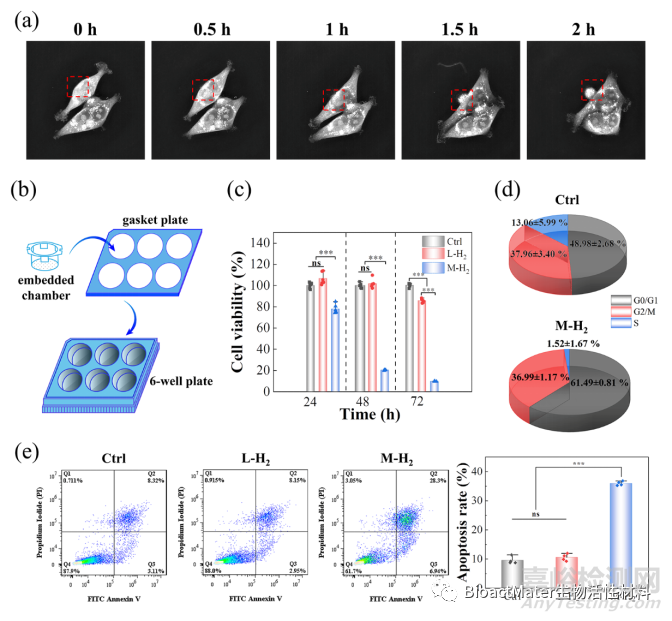
Fig.2 Hydrogen induces tumor cell apoptosis at certain critical concentrations. (a) A series of morphology images of HCT116 cells from a live cell imaging microscope. (b) Schematic illustration of H2-emitting device. (c) The cell viability of HCT116 cells with different treatments. (d) The cell cycle of the HCT116 cells in the Ctrl and M-H2 groups; and (e) flow cytometry of cell apoptosis after different treatments and the corresponding apoptosis rate was statistically quantified. L-H2 and M-H2 groups represent the low and medium concentrations of H2 in the culture medium, respectively. The ctrl group represents no H2-treatment.
综合通过MSigDB、KEGG和GO三个数据库分析mRNA转录组测序结果,氢分子通过上调p53基因的表达,来调节溶酶体-线粒体诱导细胞凋亡 (Fig. 3)。
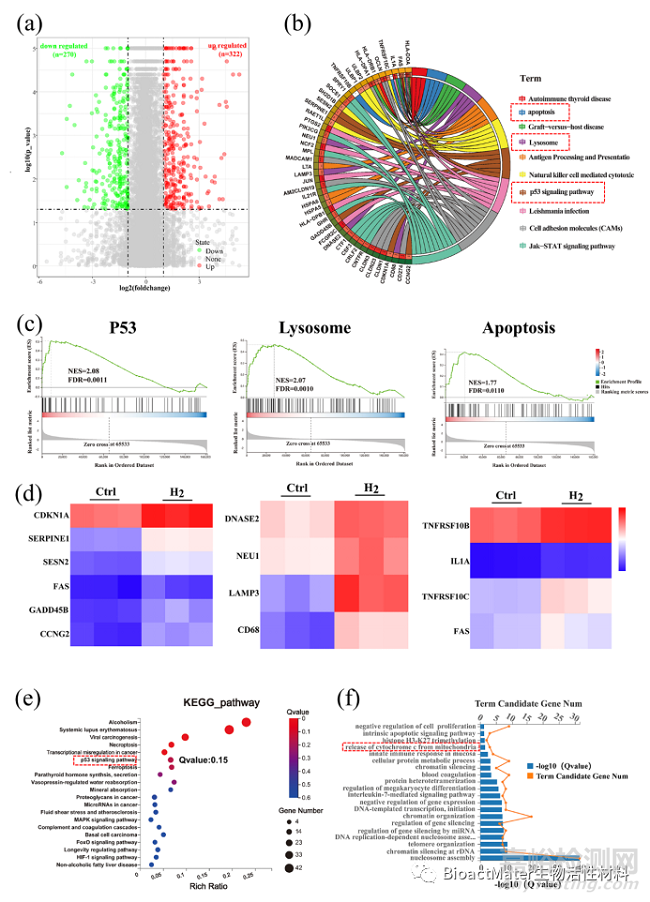
Fig.3 Hydrogen regulated gene expressions related to the P53-mediated apoptosis signaling pathway. (a) A volcano plot showing up/down-regulated genes after M-H2 treatment vs. the Ctrl group. Genes with an absolute fold change of >2 and a P value of <0.05 are highlighted in green and red color separately, denoting the down- and up-regulated genes, respectively. (b) Circular visualization of the gene-annotation enrichment analysis after gene set enrichment analysis (GSEA) was conducted using the Molecular Signatures Database (MSigDB). (c) The gene set enrichment analysis of the P53, lysosome and apoptosis pathway. (NES: normalized enrichment score). (d) A heat map of differentially expressed genes associated with three apoptotic pathways after H2 treatment, a fold change of >2 and a p value of <0.05 compared to the Ctrl group was analyzed. (e, f) An analysis of the enriched differentially expressed genes using the Kyoto Encyclopedia of Genes (KEGG) and Genomes (GO) database.
接下来,测序结果提示的作用通路相关蛋白表达,通过western blot和免疫荧光实验进行测试,来验证氢分子调控p53诱导结直肠肿瘤细胞凋亡通路的有效性 (Fig. 4)。结果发现,氢分子提高抑癌基因p53的表达水平,促使线粒体的膜电位去极化,激活细胞自噬产生凋亡小体,清除了肿瘤细胞内的活性氧,最终导致了肿瘤细胞的凋亡 (Fig. 5)。
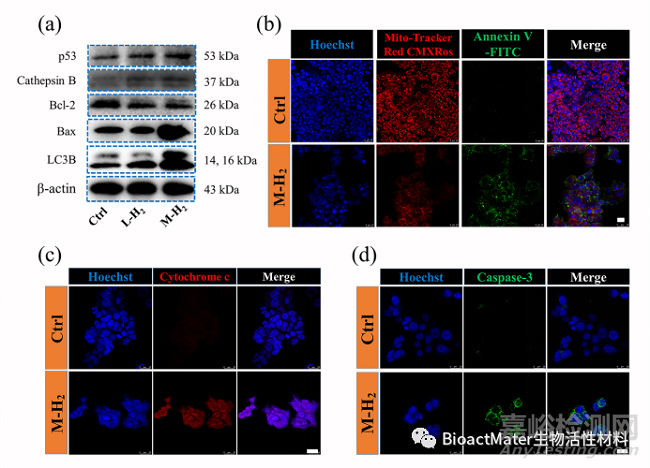
Fig. 4 The up-regulated p53 activates the mitochondrial apoptosis pathway by hydrogen. (a) The p53, cathepsins, Bax, Bcl-2 and LC3B expression after M-H2, L-H2 and Ctrl treatment, respectively. (b) Confocal fluorescence images of mitochondria membrane potential and apoptosis of HCT116 with or without H2 treatment. The mitochondria membrane potential was stained by Mito-Tracker Red CMXRos (red), apoptosis cells were stained by Annexin V-FITC (green) and the nuclei were stained by Hoechst 33342 (blue). The scale bar is 25 μm. (c) The analysis of cytochrome C expression in HCT116 cells using the APC-conjugated cytochrome C antibody. Scale bar: 25 μm. (d) The analysis of the caspase-3 expression in HCT116 cells using FITC-conjugated cleaved caspase-3 antibody. Scale bar: 25 μm.
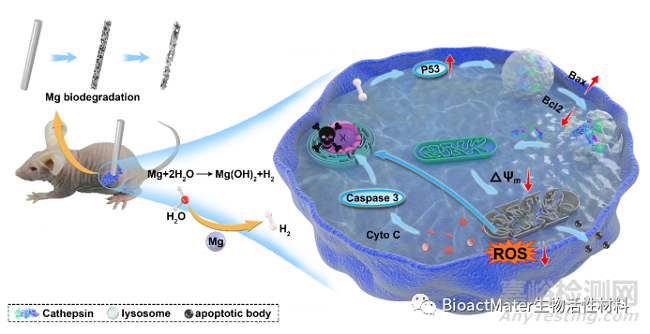
Fig. 5 Schematic illustration of the anti-tumor effect of H2 from biodegradable Mg
基于体外细胞学实验研究结果,进一步开展动物体内实验验证氢气的抗肿瘤效果,并比较镁植入物肿瘤周围原位释放氢气和吸入氢气这两种氢气输送方式对肿瘤的抑制率。结果显示,镁植入物在体内均匀地释放氢气,通过控制镁与体液的暴露面积,实现肿瘤周围可控的溶解氢气浓度影响肿瘤治疗。在植入24天后,当镁与体液接触面积为51.9 mm2时,镁局部释放氢气比全身性吸入氢气表现出更高的肿瘤抑制效果,肿瘤体积降低了27.0% (Fig. 6)。
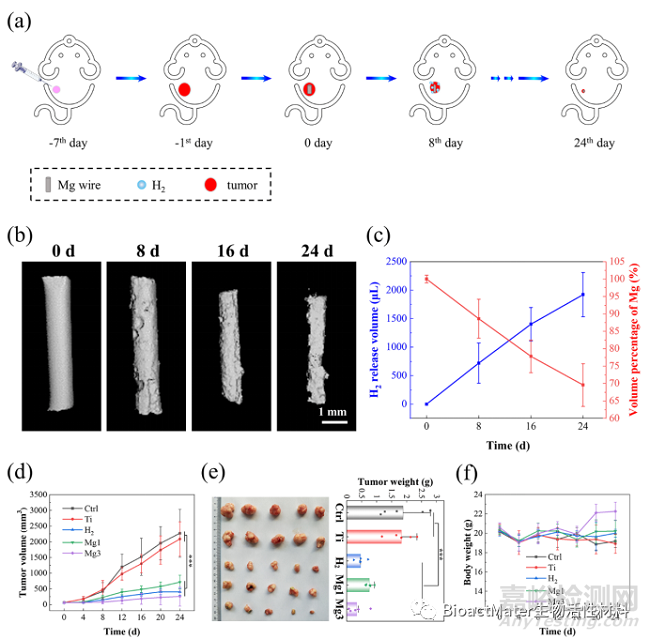
Fig. 6 Hydrogen released from Mg wires inhibits tumor growth in vivo. (a) The anti-tumor evaluation of the H2 therapies through Mg degradation on tumor-bearing mice. (b) Micro-CT images of Mg wires after 0 d, 8 d, 16 d, and 24 d implantation in tumor tissue; (c) Evolution of hydrogen released and wire volumes in tumor-bearing mice after 8 d, 16 d and 24 d implantation. (d) Growth curve of tumor volume after different treatments. (e) The HCT116 tumor weight and the corresponding representative pictures of tumors at the end of the experiment. (f) Bodyweight change after different treatments.
通过对不同处理组的肿瘤组织进行TEM超微组织观察、H&E染色、TUNEL染色及caspase-3染色,镁局部释放氢气表现出良好的体内诱导肿瘤细胞凋亡效果 (Fig. 7)。
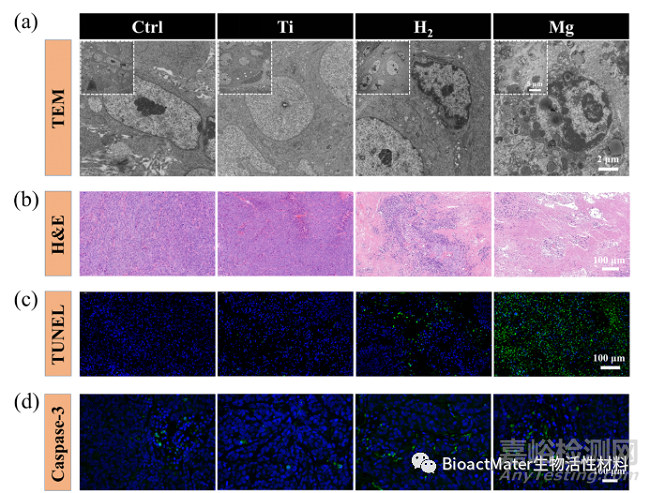
Fig. 7 Hydrogen induces tumor tissue apoptosis in tumor-bearing mice. (a) TEM photos of the tumor tissues at various scales; (b) H&E images of tumor tissues dissected from each group after different treatments on day 24; (c) Apoptosis was analyzed by TUNEL staining in tumor tissue after 24 days of treatment; (d) Confocal laser scanning microscopy of caspase-3 staining in tumor tissues after different treatments.
总之,本研究发现可降解镁金属局部释放氢气呈浓度依赖性诱导结直肠肿瘤细胞HCT116凋亡。通过控制镁的降解速率来调节溶解在体液中的氢的浓度,例如改变介质pH值或暴露于溶液的表面积。氢分子上调肿瘤抑制因子p53表达,通过溶酶体-线粒体介导的凋亡信号通路诱导肿瘤细胞凋亡。荷瘤小鼠体内的镁植入实验也证实了其原位释放氢气的抗肿瘤治疗效果。这些结果表明,医用镁植入器械(如吻合钉)控制释放氢气对抑制结直肠肿瘤具有很大的应用潜力。

来源:BioactMater生物活性材料


