您当前的位置:检测资讯 > 科研开发
嘉峪检测网 2022-09-25 22:25
TestMethod Assessment for Bioburden and Endotoxin
Swab Recovery Method Assessment
擦拭回收率方法评估
An equipment rinse is performed using a solvent that doesnot interfere with recovery. The swabbing technique, although it has a specialadvantage over the rinse sampling method, has the major disadvantage of a lowrecovery of collected bioburden.The reason for low recovery is related to the swab fiber matrix, which hindersthe release of
microbial cells.
设备淋洗使用不干扰回收的溶剂。擦拭技术虽然比淋洗取样法有独特的优点,但其主要缺点是收集到的生物污染物回收率低。其原因与棉签纤维基质阻碍微生物细胞释放有关。
The recovery study results should be assessed by the QC laboratory when reviewing cleaning validation and routine microbiological data. If the recovery results are low, then an RCF should beapplied to the final test results to compensate forsampling method limitations and to determine if the acceptance criteria weremet.
在审查清洁验证和日常微生物数据时,应由QC实验室对回收试验结果进行评估。如果回收率低,则应在最终试验结果中使用回收校正因子RCF,以补偿取样方法的局限性,并确定是否满足接受标准。
For standard bioburden recovery, after swabbing is complete typically the swab is either streaked onto an agar mediumor transferred into a liquid medium, vortexed for about 30 s and then theliquid sample preparation is tested by pour-plate or membrane filtration method[37].
对于标准的生物负荷回收,在擦拭完成后,通常既要将擦拭棉签划到琼脂培养基上又要转移到液体培养基中,离心约30 s,然后通过倾注平板或膜过滤法测试制备好的液体样品[37]
Usinga commercial swab kit is recommended. There are many differentcommercially available swabs that can be used, especially for surface sampling.Most swab wetting solutions contain an emulsification and neutralizing bufferto neutralize cleaning agents that may inhibit microbial growth. Theemulsification solution extracts the microorganismsfrom the swab material and disperses them into the solution. This allows therecovery of any microorganismsexposed to specific cleaning agents
建议使用商用棉签。有许多不同的商用棉签可以使用,特别是表面取样。大多数擦拭用润湿溶液有乳化和中和缓冲液,以中和可能抑制微生物生长的清洁剂。有乳化作用的溶液从棉签材料中提取微生物并分散到溶液中。这样就可以回收暴露到特定清洁剂中的任何微生物。
It should be noted that typicalrecovery specifications applied to chemical cleaning validation are not appliedto microbiological recovery. For bioburden limits, a 50% recovery isoutstanding; usually recoveries obtained are around 5%–20%, with some companiesstating only “growth” as the recovery requirement. The bioburden recoveryresults for some materials (porous) can even be less. However, the methoddescribed in this Guide should assist in getting recovery rates greater than50%.
应注意,通常用于化学清洁验证的回收率标准不适用于微生物回收。对于生物负荷限度,50%的回收率已经是很好的了;通常回收率在5%-20%左右,有些公司只将“生长”作为回收要求。对于某些材料(多孔材料)的生物负荷回收结果甚至可能更少。然而,本指南中描述的方法应有助于使回收率大于50%。
Consider that there is one factoraffecting these recoveries that is normally not an issue in chemical cleaning validation: the survival of the microorganisms.
考虑到有一个因素会影响这些回收率,这在化学清洁验证中通常不是一个问题:即微生物的存活率。
The following data is required to calculate the limits:
计算限值需要以下数据:
Worst-case product/equipment train combination
产品/设备链的组合的最差情况
Lowest specification for productcontamination (103CFU/g aerobicbacteria, 102 CFU/g molds and yeasts) (solids)[46, 109]
产品污染的最低标准(需氧菌:1000CFU/g,霉菌和酵母菌:100 CFU/g)(固体)【46,109】
10% contribution to the totalcount by the equipment (90% of the contamination coming from unknown sources)
设备污染占产品总微生物限度的10%(90%的污染物来自未知来源)
0.1 safety factor
0.1安全因子
Per current versions of USP21 [46] and Ph. Eur. [109], the microbial requirements for non-sterilepharmaceutical DPs are:
根据当前版本的USP21[46]和EP[109],非无菌药物DPs的微生物要求为:
Control of the total bioburden
总生物负荷控制
Elimination of USP indicator andobjectionable microorganisms
控制USP控制菌和致病菌
Cultures usually need to be heavilydiluted prior to plating; otherwise, instead of obtaining single colonies thatcan be counted, a so-called “lawn” forms: thousands of colonies lying over eachother. Additionally, plating is the slowest method. Most microorganisms need atleast 24 hours to form visible colonies. The enumeration of colonies on agar plates can be greatly facilitated by using colonycounters.
培养物通常需要在注板前进行足够稀释;否则,就不会获得可计数的独立菌落,而是形成所谓的“菌苔”:数千个菌落相互重叠。另外,注板是最慢的方法。大多数微生物需要至少24小时才能形成可见的菌落。使用菌落计数器可大大方便琼脂平板上菌落的计数。
To quantify the number of microorganisms in a culture,the microorganism is plated on a Petri dish with growth medium. If the microorganism cellsare efficiently distributed (non-spreaders) on the plate, it is generallyassumed that each microorganism cell will give rise to a single colony or CFU.The colonies are then counted and, based on the known volume of culture that was spread on the plate,the cell concentration calculated.
为了量化培养物中微生物的数量,将微生物注入有促生长培养基的培养皿上。如果微生物细胞有效地分布在平板上(非铺展剂),通常假设每个微生物细胞会产生一个菌落或CFU。然后对菌落进行计数,并根据在平板上铺展的已知培养体积计算细胞浓度。
The primary technique in countingcolonies is to count each colony dot once. One approach is to set the Petridish on a grid background and count thecolonies in each grid cell, moving in a methodical pattern through all thecells. Marking counted colonies on the back of the Petri dish can also be ahelpful approach. Generally, you will need to count at least three plates; onlyuse plates containing 30 to 300 colonies to make robust inferences. Plates withcolonies that are too numerous to count or with too few colonies need to bere-plated from a new dilution. Another approach is to use a colony counter for inspection of allplates to ensure a consistent counting background.
菌落计数的主要技术是每个菌落点计数一次。一种方法是将培养皿设置在网格背景上,计算每个网格单元中的菌落数,在所有的网格中有条不紊地移动。在培养皿背面标记已计数的菌落也是一种有用的方法。一般来说,你需要数至少三个平板;只使用含有30到300个菌落的平板来做出有力的推断。菌落太多而无法计数或菌落太少的平板需要从一个重新稀释的溶液中重新进行注板。另一种方法是使用菌落计数器检查所有平板,以确保计数背景一致。
Endotoxin Surface Sampling
内毒素表面取样
Bacterial endotoxins are typicallydetected from swab and rinse samples using the LAL method. If swabs are used,extraction methods should be developed prior to processing the samples.
细菌内毒素通常使用LAL法从擦拭和淋洗样品中检测。如果使用棉签,应在处理样品之前开发提取方法。
Equipment swabbing needs to beperformed by qualified personnel, using sterile swabs made from materials thatdo not interfere with the test. There are several types of swabs used tomonitor flat or hard-to-reach surfaces such as the bottom of a tank, O-rings,traps, transfer lines, and U-bends, although rinse samples are much easier totake.
设备擦拭需要使用由不干扰试验的材料制成的无菌棉签并由经确认的人员来实施,有几种类型的棉签用于监测平坦或难以触及的表面,如储罐底部、O形环、存水弯、输送管线和U形弯管,尽管冲洗样品更容易获得。
“The Bacterial Endotoxins test can be performed by the kineticturbidimetric, kinetic chromogenic, or gel-clot test methods. However, thekinetic test methods have significant advantages over the gel-clot test.”
细菌内毒素检查可采用动态浊度法、动态显色法或凝胶凝块试验法。然而,动态测试法比凝胶凝块试验法有显著优势。”
“A BET involves analyzing the liquid sample or sample extract using LimulusAmebocyte Lysate (LAL). LAL is a reagent made from the blood of the horseshoecrab. In the presence of bacterial endotoxins, the lysate reacts to form a clotor cause a color change depending on the technique. The test sample is comparedto a standard curve made from known endotoxin concentrations. Alltests are performed in at least duplicate. A positive product control andnegative control are included as part of each assay.”
BET涉及使用鲎试剂(LAL)分析液体样品或样品提取物。鲎试剂是一种由鲎血制成的试剂。在存在细菌内毒素的情况下,溶解物会根据机理反应形成凝块或引起颜色变化。将测试样品与已知内毒素浓度制得的标准曲线进行比较,双样检测。阳性品对照和阴性对照作为每次分析的一部分。”
“It is required to demonstrate that the test sample does not interfere withthe ability to detect endotoxins. This is accomplished with the positiveproduct control (also called the spike recovery) for the kinetic test methods,and with a separate inhibition and enhancement assay for thegel-clot method.” [110]
“需要证明测试样品不干扰检测内毒素的能力。这可以通过动力学检测方法的阳性对照(也称为加样回收)和凝胶凝块法的单独抑制和增强试验来实现。
Microbiological(Virus, Mycoplasma, and TSE) Studies to Support Cleaning Requirements
支持清洁要求的微生物(病毒、支原体和TSE)研究
The challenges for cleaningvalidation in biopharmaceutical and biological processes are somewhat differentfrom the traditional validation of small-molecule chemical residues. In thebiopharmaceutical industry, the main issue is microbiological contamination including viral,mycoplasma, bacteria, fungi, and other biological residues, and almost all of these can be overcome byusing sodium hydroxide and other alkaline solutions in the cleaning process.
生物制药和生物工艺中清洁验证的挑战与传统的小分子化学残留验证有所不同。在生物制药工程中,主要的问题是微生物污染,包括病毒、支原体、细菌、霉菌和其他生物残留物,几乎所有这些都可以通过在清洁过程中使用氢氧化钠和其他碱性溶液来去除。
Most manufacturing facilitiesoutsource the cleaning method/cycle development activities that involvepathogenic or non-flora type organisms due to the inherent challenges andpossible facility and/or laboratory contamination risks. The data generated from these studies supports thedevelopment and validation of the cleaning cycles including types of cleaningagents used, sampling methods, and other relevant process requirements. It isunderstood that special biological handling, precautions, and containmentcontrols should be implemented when working with the organisms described.
由于固有的挑战和可能的设施和/或实验室污染风险,大多数制造厂将涉及致病性或非植物性有机体的清洁方法/程序开发活动外包出去。这些研究产生的数据支持清洁程序的开发和验证,包括使用的清洁剂类型、取样方法和其他相关工艺要求。众所周知,当处理上述生物体时,应实施特殊的生物处理、预防措施和控制措施
Process development and optimization in upstream processincludes various parts:
上游工艺的工艺开发和优化包括以下几个部分:
Cell line development andengineering
细胞系开发和工程
Cell clone selection
细胞克隆选择
Media and feed development
培养基和供给的开发
Bioprocess development and scale up
生物工艺开发和放大
“Reactor design, cell harvesting, process control and the correspondinganalytics can be part of the optimization process as well…. These areas areoptimized individually and focus on a robust generation of a high producttiter, high productivity and defined quality…. [Figure 8.1] schematically presents the different optimization areas andlists the most important parameters.” [111]
“反应器设计、细胞播种、过程控制和相应的分析也可以是优化工艺的一部分…。这些领域被单独优化,并专注于高产品效价、高生产率和高质量的强劲产品…。[图8.1]示意性地显示了不同的优化区域,并列出了最重要的参数。”[111]
During the laboratory scale studies,the criterion at this stage is to perform the necessary challenges to validatethe removal of specific biological agent (analytics) residues to an acceptablelevel from the process stream equipment. The use of cleaning agents should becontrolled as well as all CPPs, and should be scalable to commercial quantities
在实验室规模研究期间,该阶段的标准是执行必要的挑战,以验证从工艺流设备中清除特定生物制剂(分析)残留物至可接受水平。应控制清洁剂的使用以及所有CPP,并应可扩展到商业数量
Following process development, process characterization,process transfer, and set up, GMP production takes place in combination withcleaning validation. Technical data from laboratory scale studies should betransferred to the process development group. When scaling up cleaningprocesses from laboratory scale studies (virus removal, etc.) to process developmentoptimization, it is important to maintain some degree of control of specificcleaning parameters such as cleaning solutionconcentration, cleaning methodology, and other factors [111].
在工艺开发、工艺特性、工艺转移和建立之后,GMP生产与清洁验证相结合。实验室规模研究的技术数据应移交给工艺开发小组。当清洁工艺从实验室规模的研究(病毒去除等)到工艺开发优化放大的过程中,保持对特定清洗参数(如清洗溶液浓度、清洗方法和其他因素)的某种程度的控制是很重要的[111]。
Figure 8.1: Optimization Areas and Parameters in Upstream Processing
上游工艺的优化区域和参数
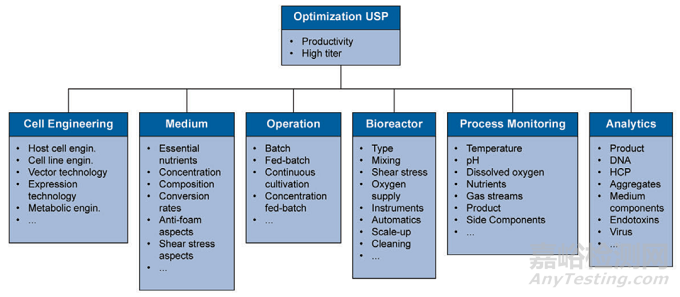
Additional information can be found“A guide to planning your Cleaning Validation Study,” by BioReliance [112].
更多信息可以在BioReliance的“清洁验证研究计划指南”中找到[112]。
“Theselection and evaluation of model microorganisms for cleaning validationstudies is a critical part of developing a removal/inactivationprotocol. The selection should consider the type of equipment and raw materialsused in production processes, and the model microorganisms should be knowncontaminants or appropriate related models. For example, bacterial and fungalspecies selected should be representative of environmental, human, and materialsource-derived microbial flora, and should include species of knownantimicrobial resistance. An additional factor to consider for a modelmicroorganism selection is its ability to grow as a high-titer stock in both standard microbiological and cell culture media, and its ease ofdetection in a sensitive and reliable assay…. Typical residualcontaminants that can be important for cleaning validation studies include:
“选择和评估用于清洁验证研究的模型微生物是制定去除/灭活方案的关键部分。选择时应考虑生产过程中使用的设备和原材料的类型,微生物模型应为已知污染物或合适的相关模型。例如,所选的细菌和霉菌种属应代表环境、人体和物料来源的微生物菌属,并应包括已知耐受物种。模型微生物选择需要考虑的另一个因素是其在标准微生物培养基和细胞培养基中作为高效价培养基生长的能力,并在灵敏和可靠的分析中易于检测…。清洁验证研究中重要的典型残留污染物包括:
Host-cell proteins
宿主细胞蛋白质
Lipids
脂类
DNA/host-cell nucleic acid
DNA/宿主细胞核酸
Endotoxins
内毒素
Carbohydrates
碳水化合物
Membrane/chromatography matrix leachables
膜/色谱基质浸出物
Detergents
洗涤剂
Viruses
病毒
TSEs
TSEs
Mycoplasmas, bacteria, fungi”
支原体、细菌、霉菌”

来源:GMP办公室


