您当前的位置:检测资讯 > 科研开发
嘉峪检测网 2022-07-20 12:03
近日,FDA授权销售Apollo内窥镜套筒胃成形术(ESG)和Revise系统,这是FDA授权的第一个用于内窥镜套筒胃成形术的系统,这是一种促进减肥的微创程序。在这个手术过程中,缝合装置和摄像机通过喉咙(食道)进入胃,并用于在胃内放置永久缝线,以减少胃的体积。它适用于肥胖(BMI 30-50 kg/m2)的成年人,他们无法通过更保守的措施(如饮食和锻炼)减肥或保持减肥。
FDA官网信息如下:
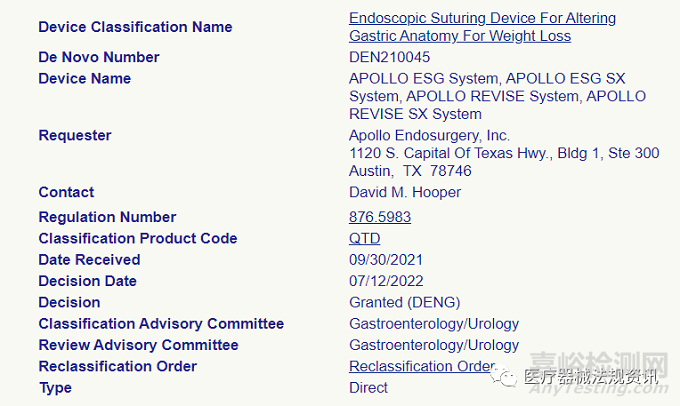
FDA授权以下此类医疗器械为II类:
Endoscopic suturing device for altering gastric anatomy for weight loss. An endoscopic suturing device for altering gastric anatomy for weight loss uses suturing to approximate gastric tissue to restrict the volume of the stomach for the intended purpose of weight loss.
此次De Novo授权的Apollo内窥镜套筒胃成形术(ESG)和Revise系统的预期用途如下:
The APOLLO ESG and ESG SX Systems are intended to be used by trained gastroenterologists or surgeons that perform bariatric procedures to facilitate weight loss by reducing stomach volume through endoscopic sleeve gastroplasty in adult patients with obesity with BMI 30 -50 kg/m2 who have not been able to lose weight, or maintain weight loss, through more conservative measures.
The APOLLO REVISE and REVISE SX Systems are intended to be used by trained gastroenterologists or surgeons that perform bariatric procedures to facilitate weight loss in adult patients with obesity with BMI 30 - 50 kg/m2 by enabling transoral outlet reduction as a revision to a previous bariatric procedure.

来源:医疗器械法规资讯


