您当前的位置:检测资讯 > 科研开发
嘉峪检测网 2025-05-19 08:21
In the realm of pharmaceutical sciences, ensuring the integrity of parenteral container closures is paramount to maintaining product sterility and safety.
在制药科学领域,确保注射用容器密封完整性对于维持产品的无菌性和安全性至关重要。
The methods employed for container–closure integrity (CCI) testing play a crucial role in this process, influencing regulatory compliance and product quality assurance. At its core, CCI testing aims to prevent the contamination ingress into pharmaceutical products, particularly in parenteral formats where sterility is non-negotiable. Microbial-ingress testing was a practical mode for evaluating a container’s ability to maintain sterility. The integrity of a closure directly impacts the product's shelf life, efficacy and, overall, patient safety. Historically, the evaluation of sterility and CCI were tightly intertwined, and microbial-ingress testing was the gold standard to establish CCI and product sterility.
用于容器密封完整性(CCI)测试的方法在这个过程中起着关键作用,影响着法规符合性和产品质量保证。从核心来看,CCI 测试旨在防止污染物进入药品,尤其是在无菌性要求不可协商的注射剂中。微生物侵入测试是评估容器维持无菌能力的一种实用模式。密封的完整性直接影响产品的保质期、功效,以及患者的整体安全。从历史角度看,无菌性和 CCI 的评估紧密相连,微生物侵入测试是确定 CCI 和产品无菌性的黄金标准。
With the advent of new drug products, delivery systems and inspection technologies, the microbial-ingress test method has lost scientific relevance for CCI.
随着新药品、给药系统和检测技术的出现,微生物侵入测试方法在 CCI 方面已失去科学相关性。
The Kirsch Study
基尔希研究
The study by Kirsch et al. (1997), often hailed as a benchmark in microbial-ingress testing, underscores the impact of submicron defects on sterility assurance. It demonstrates convincingly that even small defects can compromise the integrity of container closures, potentially leading to microbial ingress under specific conditions. This study was the first and only peer-reviewed article that took up the challenge of answering the question: What is the critical defect size for sterile products? (1)
基尔希等人(1997 年)的研究常被誉为微生物侵入测试的基准,强调了亚微米缺陷对无菌保证的影响。该研究令人信服地表明,即使是微小的缺陷也会损害容器密封的完整性,在特定条件下可能导致微生物侵入。这是第一篇也是唯一一篇经过同行评审的文章,承担起回答这个问题的挑战:无菌产品的关键缺陷尺寸是多少?(1)
One of the significant criticisms of microbial-ingress testing lies in its probabilistic nature and the lack of a globally standardized methodology. Unlike deterministic methods that provide clear, pass/fail outcomes based on measurable parameters, microbial-ingress tests can vary significantly depending on testing conditions and interpretations. This variability raises concerns about the consistency and reliability of results across different testing laboratories and scenarios.
对微生物侵入测试的一个重大批评在于其概率性质以及缺乏全球标准化方法。与基于可测量参数提供明确的通过 / 不通过结果的确定性方法不同,微生物侵入测试会因测试条件和解释的不同而有显著差异。这种可变性引发了对不同测试实验室和场景下结果的一致性和可靠性的担忧。
Kirsch et al. established several important considerations related to CCI and the microbial ingress test. It established that defects below 1 micron in size can still present a risk for microbial ingress. It also established that the risk of microbial ingress was almost certain as the defect size increased to the 10-micron mark. This microbial-ingress study was always thought to be a gold standard to assess a container’s ability to maintain sterility. Additionally, one of the most profound yet overlooked findings of the Kirsch study were these two simple takeaways:
基尔希等人确立了一些与 CCI 和微生物侵入测试相关的重要考量因素。研究确定,尺寸小于 1 微米的缺陷仍然可能存在微生物侵入的风险。同时还确定,当缺陷尺寸大至 10 微米时,微生物侵入的风险几乎是必然的。这项微生物侵入研究一直被认为是评估容器维持无菌能力的金标准。此外,基尔希研究中一个极其重要但被忽视的发现有以下两点:
Microbial ingress can occur with defects of sizes between 1 and 10 microns.
微生物侵入可能发生在尺寸在 1 到 10 微米之间的缺陷处。
Microbial ingress is not able to reliably detect defects of sizes between 1 and 10 microns.
微生物侵入测试无法可靠地检测出尺寸在 1 到 10 微米之间的缺陷。
The Kirsch study brought to light the variable method performance. If sterility was a golden standard for sterile products, but the method to establish that characteristic is highly probabilistic, that method itself is not reliable for evaluating CCI. The Kirsch study showed what microbes can achieve and the probabilistic nature of microbial-ingress test results. The microbial-ingress method has provided general clarity around the risk of ingress but does not provide the tools to mitigate that risk.
基尔希研究揭示了方法性能的可变性。如果无菌性是无菌产品的金标准,但确定该特性的方法具有高度的概率性,那么该方法本身在评估 CCI 方面并不可靠。基尔希研究展示了微生物可能造成的情况以及微生物侵入测试结果的概率性质。微生物侵入方法提供了关于侵入风险的大致情况,但没有提供减轻该风险的工具。
Despite the general clarity microbial ingress provides around the risk of ingress, the methodology behind creating the test procedure is still unclear. The industry is left without guidance for rigid parenteral products, with the only standardized method relating to single-use systems. ASTM E3251-23 Standard Test Method for Microbial Ingress Testing on Single-Use Systems, which focuses on the methodology used to establish the microbial integrity of a single-use system or to determine the maximum allowable leakage limit, directly focuses on CCI. The standard heavily weighs on arbitrary “Relevant Conditions” to create the parameters and specifications for the method and explicitly states that:
尽管微生物侵入测试在侵入风险方面提供了大致的情况,但创建测试程序背后的方法仍不明确。对于刚性注射产品,行业缺乏相关指导,唯一的标准化方法与一次性使用系统相关。ASTM E3251-23《一次性使用系统微生物侵入测试的标准测试方法》直接关注 CCI,该标准侧重于用于确定一次性使用系统微生物完整性或确定最大允许泄漏极限的方法。该标准严重依赖任意的 “相关条件” 来创建方法的参数和规格,并明确指出:
Any size defect may be forced to fail under sufficiently aggressive conditions (including a large enough sample size, high differential pressure, or high hydrostatic pressure, for example) that would not ordinarily reflect normal use conditions (2).
在足够严格的条件下(例如,足够大的样本量、高压差或高静水压),任何尺寸的缺陷都可能被强制判定为不合格,而这些条件通常不能反映正常使用条件(2)。
If a method can be forced to fail under certain conditions, it can also be forced to pass. The results of microbial-ingress testing can be influenced by operators and test-method developer bias. Although E3251 can be used for alternative containers, such as rigid parenterals (4.4.4), the limitations and bias remain.
如果一种方法在某些条件下可能被强制判定为不合格,那么它也可能被强制判定为合格。微生物侵入测试的结果可能受到操作人员和测试方法开发者偏见的影响。尽管 E3251 可用于替代容器,如刚性注射产品(4.4.4),但局限性和偏见仍然存在。
In other life-science spaces, guidance is provided by the U.S. Food and Drug Administration (FDA), where the agency recommends microbial-ingress testing for Intravascular Administration Sets Premarket Notification Submissions, but no guidance is provided on a standard procedure (3). Some laboratories have taken the lack of industry guidance into their own hands and created elaborate test procedures to challenge new packages after deeming common practices unfit for current sterility and stability requirements (4).
在其他生命科学领域,美国食品药品监督管理局(FDA)提供了相关指导,该机构建议对血管内给药装置的上市前通知提交进行微生物侵入测试,但没有提供关于标准程序的指导(3)。一些实验室自行处理行业指导缺失的问题,认为常见做法不符合当前的无菌性和稳定性要求后,创建了精心设计的测试程序来对新包装进行挑战(4)。
Position
立场
The concept of CCI and microbial ingress were once synonymous. Microbial ingress and sterility testing were the determining measures of CCI. Despite more deterministic CCI methods being developed for industry, there remains a comfort in using physical methods such as microbial ingress. While microbial ingress remains unevolved in the ability to detect defects, technology and regulation have evolved significantly.
CCI 和微生物侵入的概念曾经是同义词。微生物侵入和无菌性测试是确定 CCI 的决定性措施。尽管为行业开发了更多确定性的 CCI 方法,但使用微生物侵入等物理方法仍然存在一定的合理性。虽然微生物侵入在检测缺陷的能力方面没有发展,但技术和法规已经发生了显著变化。
The scope of CCI has evolved well beyond the protection of sterility. Parenterals need to be equally evaluated for risk of oxygen, moisture or bacterial ingress to the drug product. Today, CCI stands alone as an evaluation of the container performance, of which acting as a microbial barrier is a performance characteristic.
CCI 的范围已经远远超出了对无菌性的保护。对于注射剂,需要同样评估氧气、水分或细菌进入药品的风险。如今,CCI 独立作为对容器性能的评估,其中作为微生物屏障是其性能特征之一。
Since 1997, no further studies beyond Kirsch et al. have addressed the test method’s performance. There is no global standard for microbial-ingress testing for the performance of CCI, nor is there a unified or recommended approach. USP General Chapter <1207> Package Integrity Evaluation—Sterile Products discourages using microbial-ingress methods over deterministic alternatives for establishing CCI (5).
自 1997 年以来,除了基尔希等人的研究外,没有进一步的研究涉及该测试方法的性能。对于 CCI 性能的微生物侵入测试,没有全球标准,也没有统一或推荐的方法。美国药典通用章节 < 1207>《包装完整性评估 —— 无菌产品》不鼓励使用微生物侵入方法,而推荐使用确定性方法来确定 CCI(5)。
In 2008, the FDA published Guidance for Industry: Container and Closure System Integrity Testing in Lieu of Sterility Testing as a Component of the Stability Protocol for Sterile Products. At that time, only a few technologies existed to support deterministic CCI, and the technical importance of sterility still held regulatory prominence over other environmental contaminants. The 2008 guidance document established a regulatory position in which sterility and CCI are two separate evaluations, sterility focusing only on the sterile nature of the process and not CCI. The guidance document provides the industry with the open pathway to properly address CCI independently of microbial ingress (6).
2008 年,FDA 发布了《行业指南:容器和密封系统完整性测试,以替代无菌测试作为无菌产品稳定性方案的一部分》。当时,支持确定性 CCI 的技术很少,无菌性的技术重要性在法规方面仍高于其他环境污染物。2008 年的指导文件确立了法规立场,即无菌性和 CCI 是两个独立的评估,无菌性仅关注生产过程的无菌性质,而不涉及 CCI。该指导文件为行业提供了独立于微生物侵入来正确处理 CCI 的明确途径(6)。
Path Forward
前进方向
More than 15 years after the publication of the 2008 guidance document on CCI, the debate on how best to identify and mitigate risks is still vibrant. The concept of holistic CCI has broadened the focus for CCI through space and time to capture container–closure performance for the full life cycle of a product. The technologies and methods available for both CCI and sterility testing have evolved to improve reliability and accuracy. The products and delivery systems are more complex and have varying requirements. One thing that has not evolved is the fundamentals of biology and physics, the factors that would improve the microbial-ingress test method. Test method standards associated with microbial ingress have also not advanced from the position of being little more than a probabilistic biological indicator.
在关于 CCI 的 2008 年指导文件发布 15 年多后,关于如何最好地识别和减轻风险的争论仍然激烈。整体 CCI 的概念通过时间和空间拓宽了对 CCI 的关注范围,以捕捉产品整个生命周期的容器密封性能。用于 CCI 和无菌性测试的技术和方法已经发展,以提高可靠性和准确性。产品和给药系统更加复杂,且有不同的要求。没有发展的是生物学和物理学的基本原理,而这些因素本可以改进微生物侵入测试方法。与微生物侵入相关的测试方法标准也没有从仅仅作为概率性生物指示剂的地位取得进展。
Next-generation CCI solutions are detailed out in USP’s Chapter <1207> Packaging Integrity Evaluation-Sterile Products. The chapter outlines deterministic methods that follow a predictable, controlled process that produces a measurable result. Methods such as vacuum or pressure decay, high voltage leak detection (HVLD), helium leak detection and headspace analysis (HSA) are foundational solutions within the deterministic CCI space. These methods vary in scope and capability. Helium leak detection is highly sensitive to small leaks but requires greater sample preparation. Figure 1 highlights the risk of environmental contaminants and the associated level of leak detection by foundational CCI methods. The other methods mentioned vary in practicality, non-destructive nature, smallest detectable leak size and overall test method speed, among other characteristics. Ultimately, each method applies scientific principles to provide the most accurate and reliable measure of container closure integrity.
美国药典的 < 1207 > 章节《包装完整性评估 —— 无菌产品》详细介绍了下一代 CCI 解决方案。该章节概述了遵循可预测、可控过程并产生可测量结果的确定性方法。诸如真空或压力衰减、高压泄漏检测(HVLD)、氦泄漏检测和顶空分析(HSA)等方法是确定性 CCI 领域的基础解决方案。这些方法在范围和能力上有所不同。氦泄漏检测对小泄漏高度敏感,但需要更多的样品制备。图 1 突出了环境污染物的风险以及基础 CCI 方法的相关泄漏检测水平。提到的其他方法在实用性、非破坏性、可检测的最小泄漏尺寸和整体测试方法速度等方面存在差异。最终,每种方法都应用科学原理来提供关于容器密封完整性的最准确和可靠的测量。
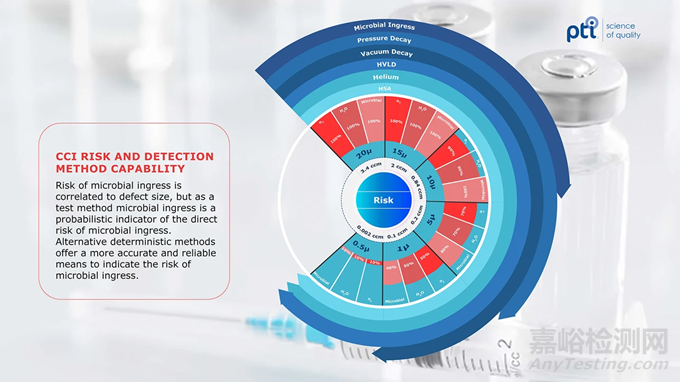
Figure 1 CCI Risk and Detection Method
图 1 容器密封完整性(CCI)风险与检测方法
There was a time and place when the microbial-ingress method belonged in the conversation of CCI. While the concern for sterility remains part of the CCI discussion, the advent of new technologies that are more capable of CCI has effectively replaced microbial ingress. The conversation on CCI was once encompassed within the evaluation of microbial-ingress testing.
曾几何时,微生物侵入方法在容器密封完整性(CCI)的讨论中占据一席之地。虽然对无菌性的关注仍是 CCI 讨论的一部分,但更能检测 CCI 的新技术的出现,已经有效地取代了微生物侵入方法。曾经关于 CCI 的讨论包含在微生物侵入测试的评估之中。
As with all pharmaceutical risk management, mitigating risk begins with fully understanding the risk. CCI is an interdisciplinary and dynamic space. Each application will present different risks, challenges and opportunities. From a regulatory standpoint, deterministic CCI test methods are gaining favor due to their robustness and ability to promptly provide actionable data.
与所有药品风险管理一样,降低风险始于充分了解风险。CCI 是一个跨学科且充满动态变化的领域。每种应用都会带来不同的风险、挑战和机遇。从监管的角度来看,确定性的 CCI 测试方法因其稳健性和能够迅速提供可采取行动的数据的能力而受到青睐。
Regulatory bodies recognize the limitations of microbial-ingress testing and increasingly emphasize the importance of deterministic testing approaches that align with current good manufacturing practices and international standards. This shift reflects a broader industry acknowledgment of the limitations of probabilistic tests in ensuring consistent product quality and safety.
监管机构认识到微生物侵入测试的局限性,并越来越强调与现行良好生产规范和国际标准一致的确定性测试方法的重要性。这种转变反映了整个行业对概率性测试在确保产品质量和安全一致性方面的局限性的更广泛认可。
Summary
总结
The industry has developed a wide variety of deterministic test methods that evaluate CCI with greater accuracy and sensitivity than ever before. Today's test methods available in the marketplace can capture defects in the microbial-ingress range with much greater reliability and accuracy. Provided the context of the microbial-ingress performance and CCI technology capability, it is difficult to argue any future for the microbial-ingress test method.
行业已经开发出了各种各样的确定性测试方法,这些方法比以往任何时候都更准确、更灵敏地评估 CCI。如今市场上可用的测试方法能够以更高的可靠性和准确性检测出微生物侵入范围内的缺陷。鉴于微生物侵入测试的性能和 CCI 技术能力的背景,微生物侵入测试方法很难有前景。
In contrast, deterministic CCI Test methods offer a more reliable and standardized approach to assessing container closure integrity. These methods, which include technologies such as Microcurrent high-voltage leak detection (HVLD), vacuum decay, and laser-based headspace analysis, provide quantitative data that directly correlate with the absence of leaks above a defined threshold. Such deterministic outcomes are crucial for regulatory compliance and provide pharmaceutical manufacturers with clear indications of product safety and quality.
相比之下,确定性的 CCI 测试方法为评估容器密封完整性提供了更可靠和标准化的方法。这些方法包括微电流高压泄漏检测(HVLD)、真空衰减和基于激光的顶空分析等技术,提供与在规定阈值以上无泄漏情况直接相关的定量数据。这样的确定性结果对于法规合规性至关重要,并为药品制造商提供了产品安全和质量的明确指示。
In conclusion, while microbial ingress testing has historically served as a benchmark for container–closure integrity assessment, its probabilistic nature and lack of standardization present significant challenges. Deterministic CCI test methods offer a compelling alternative, providing pharmaceutical scientists and manufacturers with reliable, consistent and actionable data to safeguard product integrity. As the industry continues to evolve, embracing deterministic testing approaches promises to enhance regulatory compliance, streamline quality assurance processes, and, ultimately, bolster patient safety in the realm of parenteral packaging.
总之,虽然微生物侵入测试在历史上一直是容器密封完整性评估的基准,但其概率性质和缺乏标准化带来了重大挑战。确定性的 CCI 测试方法提供了一个有吸引力的替代方案,为药品科学家和制造商提供可靠、一致且可采取行动的数据,以保障产品完整性。随着行业的不断发展,采用确定性测试方法有望提高法规合规性,简化质量保证流程,并最终在注射用包装领域增强患者安全。

来源:Internet


