您当前的位置:检测资讯 > 科研开发
嘉峪检测网 2021-10-11 23:11
Why Validation Projects Fail
为什么验证项目失败
Life Sciencescompanies are under increasing pressure to use advanced manufacturing processesand sciences to ensure that manufactured drugs meet compliance requirements ofpatient safety, product quality and data integrity. Additionally, social andpolitical pressures require them to produce drugs that are affordable to abroader section of patients across the economic ladder. To achieve thesepatient-focused outcomes, they are not only designing quality into theiroperations and systems, but they are also validating them to ensure that thesesystems and processes function as designed. This article addresses whyvalidation projects fail and result in signification cost increases.
生命科学公司面临越来越大的压力,需要使用先进的制造工艺和科学,以确保制造的药物符合患者安全、产品质量和数据完整性的合规性要求。此外,社会和政治压力要求他们生产出在经济阶梯中更广泛的患者负担得起的药物。为了实现这些以患者为中心的结果,他们不仅在操作和系统中设计质量,还需要进行验证,以确保这些系统和工艺按照设计运行。本文讨论验证项目失败并导致注册成本增加的原因。
What is Validation
什么是验证
Validation isthe documented evidence of testing to verify that computerized systems andprocesses consistently perform as designed and is “fit for use”. Sincevalidated systems must be maintained in a validated state, any changes tovalidated systems are required to be re-validated. Thus validation is arecurring cost and if not performed in an optimal manner, could significantlyincrease the Cost of Goods (COG).
验证是测试的书面证据,以证明计算机化系统和工艺始终如一地按照设计运行并"适合使用"。由于已验证的系统必须保持验证状态,因此需要对已验证系统的任何变更进行再验证。因此,验证是一种经常性成本,如果不以最佳方式执行,可能会明显增加成本(COG)。
Many validationprojects invariably overrun budgets and fail to meet schedules. This conundrumoften results in management eyeing validation costs suspiciously. Under costcontainment pressures from management, there is a tendency to cut corners whichresults in an increased compliance risks. This article provides factors thatcause validation projects to fail and how to proactively address those factorsto pre-emp failures. Some of the common observations and citations onvalidation by regulatory auditors include:
许多验证项目总是超支预算,并且不能按照预定时间完成。这个难题通常会导致管理层对验证成本产生怀疑。在管理层的成本控制压力下,存在削减开支的趋势,从而导致合规性风险增加。本文提供了导致验证项目失败的因素,以及如何主动解决这些因素以避免失败。监管检查员对验证的常见发现项和缺陷项包括:
Failure to validate a system used for regulatory purpose
未能验证受监管系统
Absence of written procedures for validation
缺乏书面的验证程序
Absent or inadequate objective evidence of validation
客观验证证据不足或不充分
Validation SOPs are not followed
未遵循验证SOP
Validation is not risk based
验证未能基于风险
Factors contributing tovalidation failure
导致验证失败的因素
Validationproject failure can be attributed to the following factors:
验证项目失败可归因于以下因素:
Unclear project objectives and methodologies
项目目标和方法不明确
Failure to engage all stakeholders consistently
未能一贯地让所有利益相关方参与其中
Absence of proactive project risk management
缺乏主动的项目风险管理
Untrained or poorly trained validation team members
验证团队成员未经培训或培训不足
Lack of project status in real time
未能实时更新项目状态
Gaps in communication
缺乏沟通
Unclear project objectives and methodologies
项目目标和方法不明确
It is important for the project sponsor to clearly define the validationproject objectives. The Project manager should develop a clear and conciseProject Execution Plan (PEP) at the start of the project and train everyproject team member on the PEP. A clear, unambiguous, validatable and concise SystemRequirements and User Requirements developed at project start by theappropriate stakeholders is also the key to a successful project. A robustValidation Project Plan (VPP) is the responsibility of the Validation lead andit should outline the scope of validation activities, the testing approach, thetesting team and their responsibilities and the acceptance criteria. Failure inplanning in areas stated above is the foundation for the failure of aValidation project.
项目负责人必须明确定义验证项目的目标。项目经理应在项目开始时制定清晰、简洁的项目执行计划(PEP),并对PEP 培训每个项目团队成员。由适当的利益相关方在项目开始时制定的清晰、明确、有效、简洁的系统需求和用户需求也是项目成功的关键。可靠的验证项目计划(VPP)是验证主管的责任,它应概述验证活动的范围、测试方法、测试团队及其职责以及接受标准。未能规划上述事项是验证项目失败的基础。
Failure toengage all stakeholders consistently
未能一贯地让所有利益相关方参与其中
Successfulprojects result from the continued engagement of a set of stakeholdersconsisting of the project sponsor. business unit executives, quality personnel,members from the IT, QC laboratory and engineering groups etc. It ensurescontinuous adjustment and alignment to meet challenges arising from changingsituations such as scope changes, human errors etc. A clear set of definedgoals and objectives, reviewed throughout the term of the project is arecommended best practice.
成功的项目来自一支利益相关方团队的持续参与。业务部门领导、质量人员、IT、QC 实验室和工程部门等。它确保持续调整和合作,以应对因范围变化、人为错误等不断变化的情况而产生的挑战。一套明确的既定目的和目标,并在整个项目期间进行审查,是推荐的最佳做法。
Absence ofproactive project risk management
缺乏前瞻性的项目风险管理
This is the mostneglected aspect of project management. During project setup and teamformation, the teams fail to proactively identify, analyze and mitigate projectrisk to scope, schedule and budget. Doing so up front will prevent projectrisks to be addressed reactively which provides management the optics of theproject team as having lost control of the project. It is prudent to developand publish a Project Risk Management Plan and educate the entire project teamon the risks.
这是项目管理中最受忽视的方面。在项目启动和团队组建过程中,团队无法前瞻性地识别、分析和降低范围、计划和预算的项目风险。预先这样做可以防止项目风险的被动处理,从而为管理层提供了项目团队对项目失控的视线。制定和发布项目风险管理计划并对整个项目团队进行风险培训是明智的。
Untrained orpoorly trained validation team members
验证团队成员未经培训或培训不足
Validation is abusiness centric activity where in a business process consisting of a sequenceof activities are used to derive an outcome e.g. a validated system. However,outcomes can be successfully achieved if we individually focus our attention tothe associated elements – such as people, team and leadership. Training peopleon the principles and methods of validation along with the associatedregulations that validation is required to satisfy is critical. Explaining tothem why they are required to do what they are required to do along with theimpact to compliance if they fail to do so is also very critical. Training themon the Validation SOPs to include Change Management etc. is also necessary, soas to avoid rework due to error.
验证是一个以业务为中心的活动,其中在由一系列活动组成的业务流程中,用于得出结果,例如:经过验证的系统。但是,如果我们将注意力集中在相关要素(如人员、团队和领导力)上,就能成功实现成果。对人员进行验证原则和方法的,以及验证要求的相关法规的培训至关重要。向他们解释,为什么他们需要执行所要求的事情,以及如果未能这样做对合规性的影响,也非常重要。还必须培训验证 SOP ,包括变更管理等,以避免因错误而从头来过。
Provision shouldalso made to establish a project onboarding training program for new projectmembers joining the team during the course of the project. Many of these risksdue to people factors may be eliminated through use of paperless validation oras the industry refers to as automation of the validation process.
还应作出规定,为项目过程中新成员加入建立上岗培训计划。多数人为因素风险可通过无纸化验证或所谓的自动化验证过程消除。
Lack of projectstatus in real time
缺乏实时项目状态
Executive management often complain that they do not have real time visibilityinto the current status of all projects. Unfortunately, they are made aware ofcritical issues when the impact on costs, timelines and scope are significantor irreversible. Since they are the final defense againstproject failure, it is critical that they be presented this visibility in realtime in a concise and clear manner such as in a dashboard format.
执行管理层经常抱怨说,他们没有实时了解所有项目的当前状态。不幸的是,当对成本、计划和范围造成重大或不可逆转的影响时,他们才意识到关键问题。因此,必须以简洁明了的方式(如看板)实时显示这些变化。
Gaps incommunication
缺乏沟通
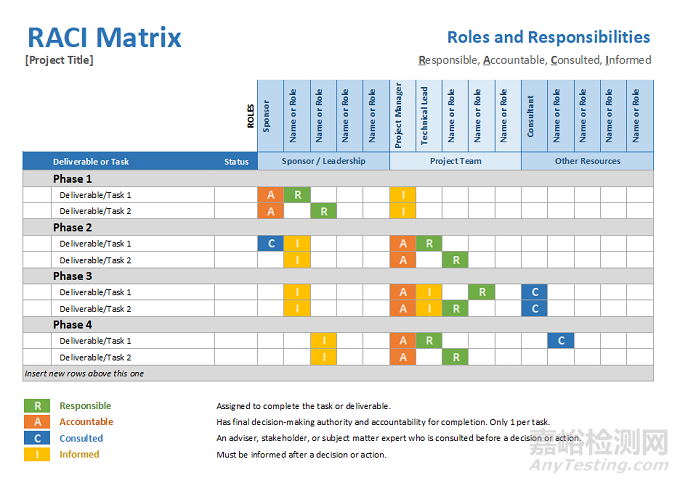
Real timevisibility into a project status is also realized through regular standingmeetings of project team members, release of meeting minutes in a timely mannerso that issues identified are satisfactorily addressed and reported out duringthe next meeting, ensuring regular attendance of all stakeholder at thesemeetings, publishing a RACI (Responsibility, Accountability, Consult, Inform)chart, identifying critical path in real time and assigning resources to movethose activities out from the critical path etc.
项目状态实时可见还可通过项目团队成员定期例会、及时发布会议纪要、在下次会议上令人满意地处理和报告已发现的问题、确保所有利益相关方定期出席这些会议、发布 RACI(谁负责(R = Responsible),谁批准(A = Accountable)、咨询谁(C = Consulted),通知谁 (I =Informed)))图表、实时确定关键路径以及分配资源以将这些活动从关键路径中移出等。
In conclusion
结论
Business expediencyrequires thatvalidation of systems and processes is a priority of executive management andthat the validation objectives are realized efficiently through appropriateplanning, risk management, preventing scope creep through planning andrequirements definition, an agreed to acceptance criteria with the projectstakeholders and ensuring that executive management including the projectsponsor has continuous visibility into project status in real time.
业务策略要求系统和工艺的验证是执行管理的优先事项,验证目标必须通过适当的计划、风险管理、通过规划和需求定义防止范围蠕变、与项目利益相关者商定接受标准以及确保包括项目申办人在内的执行管理层实时持续了解项目状态,从而有效地实现验证目标。

来源:GMP办公室


