您当前的位置:检测资讯 > 生产品管
嘉峪检测网 2025-03-10 11:45
FDA's requirements for approval of new and generic drugs and biologics are among the highest standards across the globe. Prior to FDA approval, manufacturers must prove that their products are high quality, safe, effective, and free of contamination and defects. In addition, manufacturers of non-application products, such as over-the-counter and homeopathic products, are responsible for adhering to quality standards. Pharmaceutical manufacturers, no matter where they are located, are responsible for complying with Current Good Manufacturing Practice and ensuring that only quality products reach U.S. patients.
FDA对新药、仿制药和生物制品的批准要求是全球最高的标准之一。生产商必须在FDA批准之前,证明他们的产品是高质量、安全、有效的,并且无污染和缺陷。此外,无需申请药物预批准的生产商,例如OTC和顺势疗法产品,其需要负责确保产品符合质量标准。药物生产商,无论其在何处,都要保证生产符合现行GMP的要求,并确保只有质量合格的产品才会用于美国患者。
To help ensure that high-quality drugs are sold in the U.S., CDER maintains a comprehensive quality surveillance program. A critical function of this program is testing selected drugs in FDA laboratories. This includes testing active pharmaceutical ingredients (APIs). FDA laboratories generally test drugs to standards set by the U.S. Pharmacopeia (USP), a scientific organization that sets minimum standards for the quality of medicines including:
为了确保高质量的药物在美国销售,CDER采用一个全面的质量监督程序。该程序的一个关键功能是在FDA实验室检测选定的药物。这也包括检测活性物质(APIs)。FDA实验室通常按照美国药典(USP)制定的标准检测药物,USP是一个科学组织,为药品质量制定最低标准,包括:
- Identity – is it the right drug as indicated on the label?
- 鉴别——这是标签上标明的正确药物吗?
- Assay – how much drug is there and is it consistent with the labeled amount?
- 含量——有多少药物?其与标示量一致吗?
- Impurities – are there process impurities or degradation impurities?
- 杂质——有工艺杂质和降解杂质产生吗?
- Dissolution – does the active ingredient dissolve out of the dosage unit so that the drug is available for the body to absorb?
- 溶出度——活性成分能从制剂单元里面溶出以便人体吸收吗?
CDER’s quality surveillance program includes multiple tools that complement sampling and testing, including inspections, evaluation of post-market quality reports, signal detection, and data analysis.
CDER的质量监督程序包括多种工具,以补充抽样和检测,包括审计、上市后质量报告的评估、信号检测和数据分析。
We are committed to protecting patients and consumers from unsafe, ineffective or poor-quality drugs.
我们致力于保护患者和消费者免受不安全、无效或劣质药物的侵害。
Testing
检测
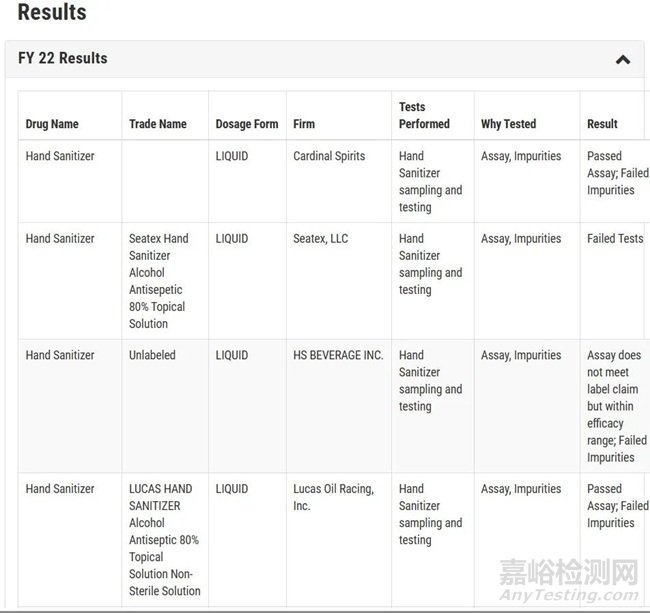
We use a risk-based approach to quality testing. This means that in cases where there is a known or likely safety, effectiveness, or quality issue with a product, FDA scientists perform specific tests for this vulnerability. As you can see in the results below, the majority of drugs FDA tests meet their specifications.
我们使用基于风险的方法进行质量检测。这意味着,在产品存在已知或可能的安全性、有效性或质量问题的情况下,FDA科学家会针对这一漏洞进行特定的检测。正如你在下面的结果中所看到的,大多数FDA检测的药物都符合其标准。
Beginning in 2018, CDER began using data analytics, including post-market quality data, to identify products that have potential quality risks. This more targeted, risk-based approach for sampling and testing has been effective in helping us identify more products per year that fail quality tests than in the years prior to 2018.
从2018年开始,CDER开始使用数据分析,包括上市后质量数据,来识别具有潜在质量风险的产品。与2018年之前相比,这种更具针对性、基于风险的抽样和测试方法有效地帮助我们每年识别出更多未通过质量检测的产品。
If drug products have unfavorable testing results, we work swiftly to protect the public from potential harm. We share information with health care professionals and consumers to help them make decisions. In addition, we alert the manufacturer of the need to take measures to correct the problem underlying the unfavorable test results. We continue to monitor the situation until the manufacturer demonstrates compliance with FDA rules and regulations.
如果药品有不合格的检测结果,我们会迅速采取行动,保护公众免受潜在的伤害。我们与医疗保健专业人员和消费者共享信息,帮助他们做出决定。此外,我们警告生产商要采取措施纠正引发不合格检测结果的潜在问题。我们会持续监测直至生产商证明其符合FDA的法规要求。
具体的检测结果可见下方列表:

来源:Internet


