您当前的位置:检测资讯 > 科研开发
嘉峪检测网 2021-06-17 09:53
受贻贝的粘附性能与关节软骨的润滑性能启发,本研究采用无规共聚法合成了多巴胺-磷酰胆碱聚合物(DMA-MPC),并利用多巴胺辅助共沉积的方式在钛合金(Ti6Al4V)表面构建了仿生自吸附聚合物涂层,获得了兼具增强润滑和抑菌粘附的材料,进而基于原子力显微镜(AFM)从微观层面研究了细菌与涂层间的相互作用,提出了基于水合斥力的抑菌机理。
01研究内容简介
医用植入物在使用过程中面临的关键挑战之一是有限的表面润滑和细菌生物膜的形成,这可能导致严重的组织损伤及一系列连续的并发症。在以往的研究中,医用植入物表面的润滑性能和抑菌性能没有得到很好的结合,并且大部分涂层材料的功能化过程较为复杂,也极大地影响了其临床应用。因此,设计一种温和的、新颖的表面功能化方法,同时考虑增强润滑和抑菌粘附非常重要。
在人体健康的关节软骨中,透明质酸、蛋白聚糖、润滑素及磷脂等生物大分子组成的表面水合润滑层是关节保持良好润滑性能的重要原因,基于水合润滑机理的磷酰胆碱类聚合物涂层也获得了越来越多的关注;自然界中的贻贝可通过黏附蛋白牢固地附着在多种基体表面,多巴胺是粘附蛋白成分中最常见的衍生物,具有与其相似的粘附性质。受此启发,本文设计、合成了修饰不同比例仿生自吸附聚合物涂层的Ti6Al4V@DMA-MPC,在进行润滑特性筛选后,采用多巴胺辅助共沉积的方式进一步辅助涂层的构建。此外,分别在宏观和微观层面研究了涂层改善前后的抑制蛋白、细菌粘附的特性,利用AFM力曲线模式测定了亲/疏水探针或者细菌与涂层之间的相互作用力,并分析了涂层的水合斥力抑菌机理,研究示意图如下,Fig. 1.
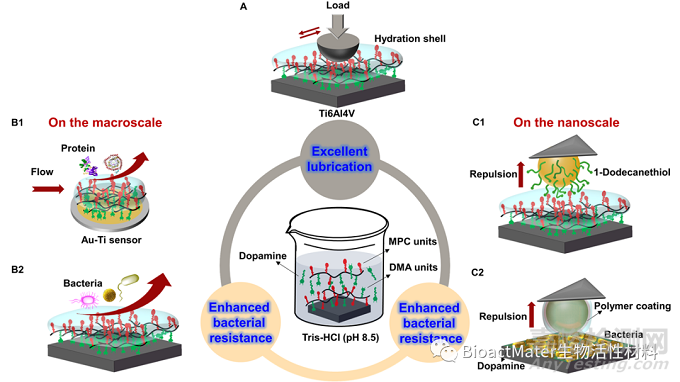
Fig. 1. Illustration showing the facile fabrication of PDA/DMA-MPC biomimetic coating for (A) excellent lubrication property based on the hydration lubrication mechanism and (B1-C2) enhanced bacterial resistance property based on hydration repulsion: (B1) quantitative evaluation of dynamic adsorption of proteins on copolymer-coated Au-Ti sensors using QCM, (B2) qualitative evaluation of bacterial resistance of copolymer-coated Ti6Al4V substrate for 24 h, and force measurements probing possible interactions between biomimetic coating and (C1) hydrophobic tips or (C2) living bacteria on the nanoscale using AFM.
常规的材料学表征证明Ti6Al4V@DMA-MPC制备成功后,利用AMF接触模式筛选出最优的比例进行后续研究。首先搭建了微流控通道,并构建了具有荧光特性的涂层基底进行流动实验,通过荧光强度的变化来考察涂层的稳定性。同时,石英晶体微天平(QCM)实验表明PDA/DMA-MPC涂层具有更优异的自吸附特性,并能获得相对更厚、更柔性的涂层,Fig. 2.
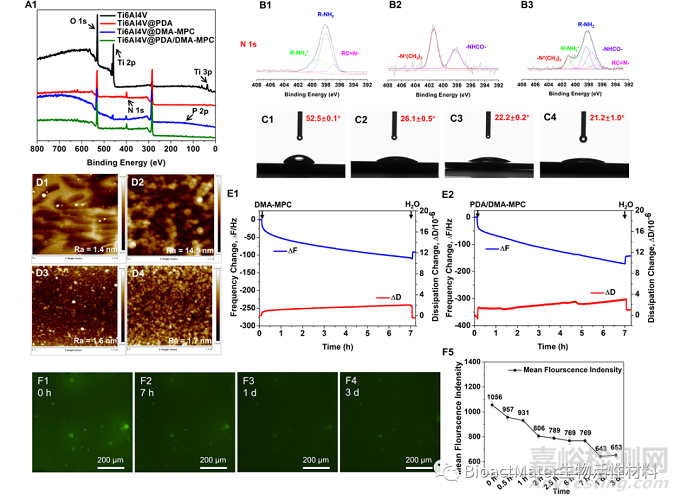
Fig. 2. (A) XPS of four different Ti6Al4V substrates. (B) High-resolution narrow spectrum of N 1s for (B1) Ti6Al4V@PDA, (B2) Ti6Al4V@DMA-MPC and (B3) Ti6Al4V@PDA/DMA-MPC. (C) WCA and (D) surface morphologies of (C1, D1) Ti6Al4V, (C2, D2) Ti6Al4V@PDA, (C3, D3) Ti6Al4V@DMA-MPC (1:4) and (C4, D4) Ti6Al4V@PDA/DMA-MPC (1:4). The Ti6Al4V substrates were treated with plasma to obtain hydroxylated surfaces. (E) Frequency and dissipation changes associated with the self-adhesive biomimetic coating for (E1) DMA-MPC and (E2) PDA/DMA-MPC. (F) Quantitatively characterized the durability of the coating by monitoring the change of fluorescence intensity in microfluidic channel under scouring experiments. The fluorescence graphs in different scouring time: (F1) 0 h, (F2) 7 h, (F3) 1 d and (F4) 3 d. (F5) The changes of fluorescence intensity in different scouring time.
在Au-Ti芯片传感器上利用QCM实时定量评价了涂层改善前后对蛋白的动态吸附性能,结果表明PDA/DMA-MPC涂层具有优异的抑制蛋白粘附性能。通过扫描电镜(SEM)观察涂层改善前后大肠杆菌的粘附情况,与细菌共培养24 h后发现,在涂层表面仅有少量粘附,表明涂层能够有效地抵抗细菌的初始粘附;与细菌共培养8 d后的定量细菌涂布实验结果表明,涂层组的抑菌效果显著,PDA/DMA-MPC涂层的抑菌率达到98%,Fig. 3.
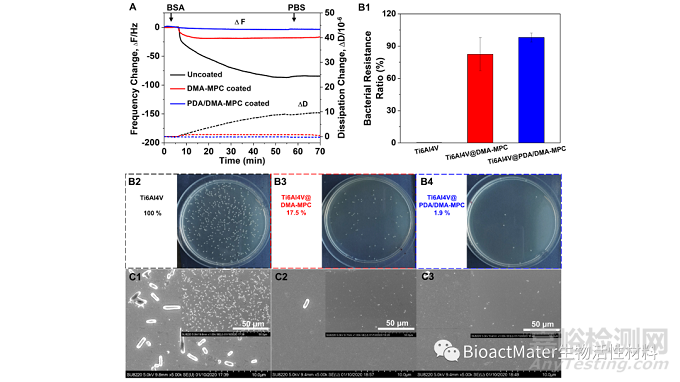
Fig. 3. (A) Frequency and dissipation changes associated with dynamic adsorption of BSA on bare and copolymer-coated Au-Ti sensors. (B) The bacterial resistance adhesion property tested by spread plate assay. (B1) The bacterial resistance ratio of different substrates. The colony pictures of (B2) bare Ti6Al4V, (B3) Ti6Al4V@DMA-MPC and (B4) Ti6Al4V@PDA/DMA-MPC, and the bacterial counts are 100 %, 17.5 % and 1.9 %, respectively. (C) Representative SEM images showing E. coli adhesion on the Ti6Al4V substrates after cultured for 24 h: (C1) bare Ti6Al4V, (C2) Ti6Al4V@DMA-MPC and (C3) Ti6Al4V@PDA/DMA-MPC.
最后,利用AFM测试了亲/疏水两种探针与涂层基底之间的相互作用力,发现疏水探针与PDA/DMA-MPC涂层在相互接近的过程中具有更强、更长程的排斥力,这说明涂层可能会在阻止蛋白质类成分的初始粘附方面发挥重要的排斥功能;同样地,PDA/DMA-MPC涂层修饰的探针与大肠杆菌基底在相互接近的过程中能够产生最强、最长程的斥力,同时在停留不同时间后,在分离过程中能够产生最小、最短程的粘附力,以上结果说明涂层优异的水合斥力屏障作用能够显著提高抑菌粘附性能,Fig. 4.
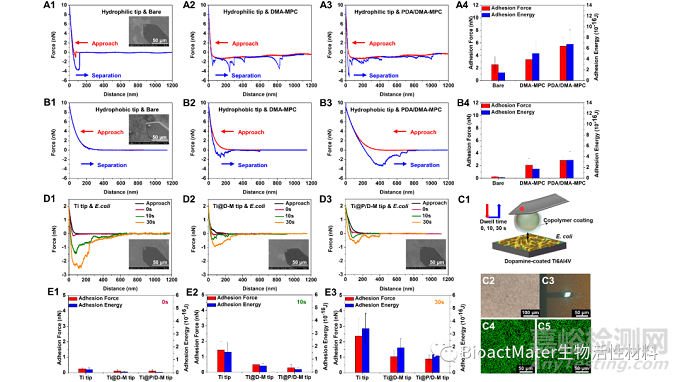
Fig. 4. Representative force-distance curves during approach and separation process in the force measurements using AFM for (A1-A3) hydrophilic tip and (B1-B3) hydrophobic tip against the bare Ti6Al4V, Ti6Al4V@DMA-MPC and Ti6Al4V@PDA/DMA-MPC substrates. The inset figures are the SEM images of the tips. (A4, B4) Statistical analysis of adhesion force and adhesion energy obtained from the separation process. (C1) Schematic illustration showing the force measurements between copolymer-coated AFM tip against bacteria-coated substrate, with different dwell times of 0 s, 10 s and 30 s. (C2) Microscopy images of the bacteria-coated substrate. (C3) Microscopy images in the force measurements between AFM tip and bacteria-coated substrate. The green fluorescence indicates that the immobilized bacteria are alive (C4) before and (C5) after the force measurements. (D) Representative force-distance curves during approach and separation process in the force measurements for (D1) Ti tip, (D2) Ti@D-M tip and (D3) Ti@P/D-M tip against bacteria-coated substrates. (E) The adhesion force and adhesion energy between three different AFM tips and bacteria-coated substrates obtained from the separation process under the dwell time of (E1) 0 s, (E2) 10 s and (E3) 30 s.
02论文第一/通讯作者简介
通讯作者:张洪玉
清华大学机械工程系摩擦学国家重点实验室副研究员,从事生物摩擦学研究,设计并研发了多种生物医用润滑材料与技术,包括微纳米颗粒、聚合物涂层以及纳米纤维膜等,用于骨关节损伤修复、医疗器械表面改性、组织粘连预防与修复等方面,承担国家自然科学基金优秀青年基金等项目10余项,以第一或通讯作者在Adv. Funct. Mater., Nano Lett., Biomaterials, Small, Nano Energy等期刊发表SCI收录论文50余篇(其中封面论文5篇),以第一发明人获授权国家发明专利10余件,参与撰写中英文专著4本,担任JWKJW生物及交叉技术领域先进仿生系统专家组助理等职务。
03资助信息
本研究得到了国家自然科学基金(52022043)、清华大学精准医学科研计划(10001020120)以及首都卫生发展科研专项(2020-2Z-40810)等项目的资助。
04原文信息
Ying Han#, Weiwei Zhao#, Yiwei Zheng, Haimang Wang, Yulong Sun, Yifei Zhang, Jing Luo, Hongyu Zhang*.
Self-adhesive lubricated coating for enhanced bacterial resistance.
Bioactive Materials 6 (2021) 2535-2545.

来源:BioactMater生物活性材料


