格氏试剂和酮反应,常用于制备叔醇,有些酮的底物,不耐受,杂质不可控,看一下下面的案例,杂质概况和解决方案。
案例概况
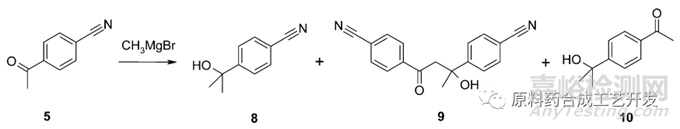
甲基溴化镁和对氰基苯乙酮5反应,制备化合物8
主要杂质信息,酮自身二聚杂质9,甲基溴化镁和氰基反应的杂质10
控制手段,优化温度,加料顺序,溶剂,没有解决杂质问题
采用温和的甲基氯化镁,也没有解决杂质问题
解决方案
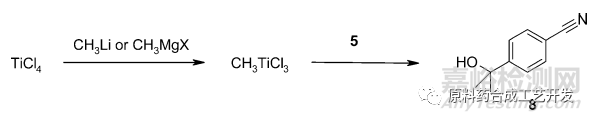
采用TiCl4和甲基锂原位产生的CH3TiCl3可以完全抑制杂质。
强亲核,弱碱性。
8kg规模,收率84%
采用甲基格氏试剂和TiCl4原位产生CH3TiCl3方法不行,反应慢。
后续步骤
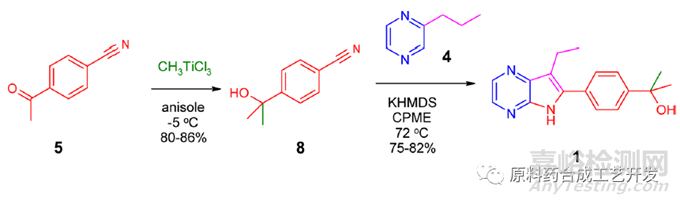
化合物8和吡嗪环4反应可得并环1
实验操作
制备化合物8
A reactor containing anisole (85 kg) was cooled to −10 to −5 °C. TiCl4 (17 kg, 89.6 mol, 1.5 equiv) was charged over 1 h while maintaining −10 to −5 °C. After stirring at −10 to −5 °C for 15 min, an orange suspension was obtained. A solution of CH3Li in cumene/2-MeTHF (61 kg, 3.2 wt %, 88.9 mol, 1.5 equiv) was charged over 100 min at −10 to −5 °C. The reddish solution was stirred at −10 to −5 °C for 35 min.
A solution of 5 (8.5 kg, 58.6 mol) in anisole (43 kg) was added over 80 min at −10 to −2 °C. The reddish solution was stirred at −5 to −2 °C for 60 min. HPLC analysis of an inprocess sample indicated that the reaction was complete (<0.5%).
The reaction was quenched by the addition of water (4.3 kg) over 30 min at −10 to 0 °C.
参考文献
Org. Process Res. Dev. 2015, 19, 806−811