您当前的位置:检测资讯 > 法规标准
嘉峪检测网 2021-10-20 15:58
10月18日,FDA在其官网发布了2021年缺陷汇总报告,其中包含FDA在2020年10月1日至2021年9月30日期间检查的2430条检查缺陷,其中药品215条,医疗器械191条,生物制品17条。
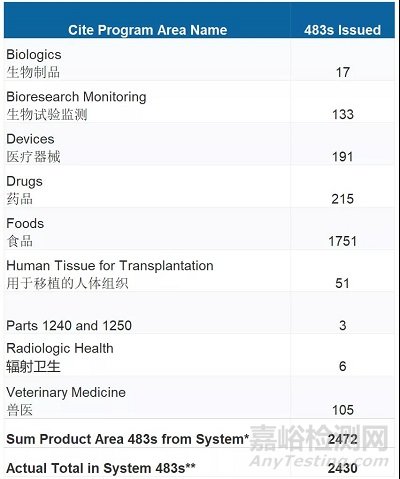
药品领域前十大缺陷如下:
1、"Theresponsibilities and procedures applicable to the quality control unit are not[in writing] [fully followed]. Specifically, ***
没有[书面]的质量控制单位的责任和程序或[没有完全遵循]。"
检查频率:80
2、"There isa failure to thoroughly review [any unexplained discrepancy] [the failure of abatch or any of its components to meet any of its specifications] whether ornot the batch has been already distributed. Specifically, ***
未能彻底审查[任何无法解释的差异][批次或其任何组分未能符合其任何标准],无论该批次是否已经放行。"
检查频率:49
3、"Thereare no written procedures for production and process controls designed toassure that the drug products have the identity, strength, quality, and puritythey purport or are represented to possess. Specifically, ***
没有书面的生产和工艺控制程序,用以确保药品具有其声称的鉴定、含量、质量和纯度。"
检查频率:44
4、"Laboratorycontrols do not include the establishment of scientifically sound andappropriate [specifications] [standards] [sampling plans] [test procedures]designed to assure that [components] [drug product containers] [closures][in-process materials] [labeling] [drug products] conform to appropriatestandards of identity, strength, quality and purity. Specifically, ***
实验室控制不包括建立科学合理和适当的[标准][规范][取样计划][检测程序],旨在确保[部件][药品容器][密封部件][中间产品][标签][成品]符合适当的鉴定、含量、质量和纯度标准。"
检查频率:40
5、"Equipmentand utensils are not [cleaned] [maintained] [sanitized] at appropriateintervals to prevent [malfunctions] [contamination] that would alter thesafety, identity, strength, quality or purity of the drug product. Specifically, ***
设备和器具未能在适当的间隔内[清洁][维护][消毒],以防止[故障][污染]改变药品的安全性、鉴定、含量、质量或纯度。"
检查频率:33
6、"Appropriatecontrols are not exercised over computers or related systems to assure thatchanges in master production and control records or other records areinstituted only by authorized personnel. Specifically, ***
未能对计算机或相关系统进行适当的控制,以确保主生产和控制记录或其他记录的更改仅由授权人员进行。"
检查频率:30
7、"Equipmentused in the manufacture, processing, packing or holding of drug products is not [of appropriate design][of adequate size] [suitably located] to facilitate operations for its[intended use] [cleaning and maintenance]. Specifically, ***
用于生产、加工、包装或保存药品的设备未能(设计适当)[适当尺寸][适当安装],以适用其[预期用途][清洁和维护]的操作。"
检查频率:25
8、"Proceduresdesigned to prevent microbiological contamination of drug products purportingto be sterile are not [established] [written] [followed]. Specifically, ***
未能[建立][书面][遵循]用以防止无菌药品的微生物污染的程序。"
检查频率:22
9、"Routine[calibration] [inspection] [checking] of [automatic] [mechanical] [electronic] equipment is not performedaccording to a written program designed to assure proper performance. Specifically, ***
未能按照用以确保适当性能的书面程序对[自动][机械][电子]设备进行日常[校准][检查][核验]。
检查频率:19
10、Employees arenot given training in [the particular operations they perform as part of theirfunction] [current good manufacturing practices] [written procedures requiredby current good manufacturing practice regulations]. Specifically, ***
员工未接受[他们作为职能的一部分执行的特定操作][CGMP][CGMP要求的书面程序]的培训。
检查频率:18

来源:Internet


